This is a preprint.
FORWARD GENETICS IN C. ELEGANS REVEALS GENETIC ADAPTATIONS TO POLYUNSATURATED FATTY ACID DEFICIENCY
- PMID: 40501978
- PMCID: PMC12157585
- DOI: 10.1101/2024.11.08.622646
FORWARD GENETICS IN C. ELEGANS REVEALS GENETIC ADAPTATIONS TO POLYUNSATURATED FATTY ACID DEFICIENCY
Update in
-
Forward genetics in C. elegans reveals genetic adaptations to polyunsaturated fatty acid deficiency.Elife. 2025 Jul 8;13:RP104181. doi: 10.7554/eLife.104181. Elife. 2025. PMID: 40627529 Free PMC article.
Abstract
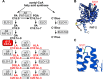
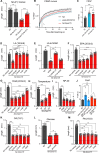
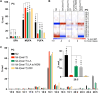
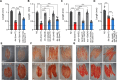
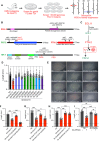
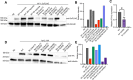
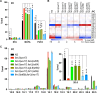
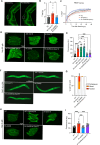
Polyunsaturated fatty acids (PUFAs) are essential for mammalian health and function as membrane fluidizers and precursors for signaling lipids though the primary essential function of PUFAs within organisms has not been established. Unlike mammals who cannot endogenously synthesize PUFAs, C. elegans can de novo synthesize PUFAs starting with the Δ12 desaturase FAT-2 which introduces a second double bond to monounsaturated fatty acids to generate the PUFA linoleic acid. FAT-2 desaturation is essential for C. elegans survival since fat-2 null mutants are non-viable; the near-null fat-2(wa17) allele synthesizes only small amounts of PUFAs and produces extremely sick worms. Using fluorescence recovery after photobleaching (FRAP), we found that the fat-2(wa17) mutant has rigid membranes and can be efficiently rescued by dietarily providing various PUFAs, but not by fluidizing treatments or mutations. With the aim of identifying mechanisms that compensate for PUFA-deficiency, we performed a forward genetics screen to isolate novel fat-2(wa17) suppressors and identified four internal mutations within fat-2, and six mutations within the HIF-1 pathway. The suppressors increase PUFA levels in fat-2(wa17) mutant worms and additionally suppress the activation of the daf-16, UPRer and UPRmt stress response pathways that are active in fat-2(wa17) worms. We hypothesize that the six HIF-1 pathway mutations, found in egl-9, ftn-2, and hif-1 all converge on raising Fe2+ levels and in this way boost desaturase activity, including that of the fat-2(wa17) allele. We conclude that PUFAs cannot be genetically replaced and that the only genetic mechanism that can alleviate PUFA-deficiency do so by increasing PUFA levels.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
