This is a preprint.
Kinetic Modeling of Covalent Inhibition: Effects of Rapidly Fluctuating Intermediate States
- PMID: 40502015
- PMCID: PMC12154943
- DOI: 10.1101/2025.05.28.656658
Kinetic Modeling of Covalent Inhibition: Effects of Rapidly Fluctuating Intermediate States
Update in
-
Kinetic Modeling of Covalent Inhibition: Effects of Rapidly Fluctuating Intermediate States.J Chem Inf Model. 2025 Aug 12. doi: 10.1021/acs.jcim.5c00952. Online ahead of print. J Chem Inf Model. 2025. PMID: 40795124
Abstract
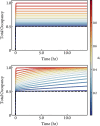
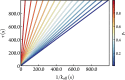
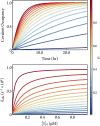
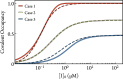
There is increasing interest in the discovery of small-molecule inhibitors that form covalent bonds with their targets for therapeutic applications. Nevertheless, identifying clear rational design principles remains challenging because the action of these molecules cannot be understood as common noncovalent inhibitors. Conventional kinetic models often reduce the binding of covalent inhibitors to a two-step irreversible process, overlooking rapid complex dynamics of the associated unlinked inhibitor before the formation of the covalent bond with its target. In the present analysis, we expand the intermediate state into two conformations-reactive (E.I) and nonreactive (E..I). To illustrate the consequences of such simplification, the expanded kinetic model can be reduced to an effective two-step scheme expressed in terms of the equilibrium probability of the unlinked inhibitor to form either conformation. A mass-action-based numerical workflow is implemented to simulate time-dependent kinetics, overcoming the common limitations of empirical models. The numerical workflow helps relate microscopic states observed in molecular dynamics simulations to macroscopic observables like and the apparent rate of covalent inhibition, showing the impact of transient intermediates on dissociation rates and potency. The proposed framework refines the interpretation of dose-response data, aiding medicinal chemists in optimizing covalent inhibitors and provides a quantitative platform for relating molecular conformational distributions to empirical parameters.
Figures
Similar articles
-
Kinetic Modeling of Covalent Inhibition: Effects of Rapidly Fluctuating Intermediate States.J Chem Inf Model. 2025 Aug 12. doi: 10.1021/acs.jcim.5c00952. Online ahead of print. J Chem Inf Model. 2025. PMID: 40795124
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
References
-
- Baker B. R. Factors in the Design of Active-Site-Directed Irreversible Inhibitors. Journal of Pharmaceutical Sciences 1964, 53, 347–364. - PubMed
-
- Singh J.; Petter R. C.; Baillie T. A.; Whitty A. The Resurgence of Covalent Drugs. Nat Rev Drug Discov 2011, 10, 307–317. - PubMed
-
- Mah R.; Thomas J. R.; Shafer C. M. Drug Discovery Considerations in the Development of Covalent Inhibitors. Bioorg Med Chem Lett 2014, 24, 33–39. - PubMed
-
- Bauer R. A. Covalent Inhibitors in Drug Discovery: From Accidental Discoveries to Avoided Liabilities and Designed Therapies. Drug Discov Today 2015, 20, 1061–1073. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
