This is a preprint.
Temporally-segregated dual functions for Gfi1 in the development of retinal direction-selectivity
- PMID: 40502160
- PMCID: PMC12157548
- DOI: 10.1101/2025.06.03.657700
Temporally-segregated dual functions for Gfi1 in the development of retinal direction-selectivity
Abstract
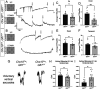
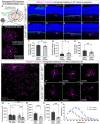
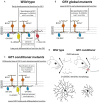
There is great diversity in retinal ganglion cells subtypes in the mouse retina, but little is known about the molecular factors required to generate this subtype diversity. Here, we identify the transcription factor Gfi1 as a conserved driver of differentiation specifically in two downward-tuned direction selective ganglion cells (DSGCs). Further, we describe a post-differentiation role for Gfi1 in regulating dendritic development of Down ON-type DSGCs crucial for their ability to detect downward motion. These results define novel temporally-segregated dual functions for Gfi1 in the development of retinal direction-selective circuits, and they provide a framework for understanding fundamental mechanisms underlying direction-selectivity in the retina.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Similar articles
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Surgical interventions for bilateral congenital cataract in children aged two years and under.Cochrane Database Syst Rev. 2022 Sep 15;9(9):CD003171. doi: 10.1002/14651858.CD003171.pub3. Cochrane Database Syst Rev. 2022. PMID: 36107778 Free PMC article.
-
Interventions for interpersonal communication about end of life care between health practitioners and affected people.Cochrane Database Syst Rev. 2022 Jul 8;7(7):CD013116. doi: 10.1002/14651858.CD013116.pub2. Cochrane Database Syst Rev. 2022. PMID: 35802350 Free PMC article.
References
-
- Vaney D.I., Sivyer B., and Taylor W.R., Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci, 2012. 13(3): p. 194–208. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
