This is a preprint.
OGDHL regulates tumor growth, neuroendocrine marker expression, and nucleotide abundance in prostate cancer
- PMID: 40502162
- PMCID: PMC12154886
- DOI: 10.1101/2025.05.28.656673
OGDHL regulates tumor growth, neuroendocrine marker expression, and nucleotide abundance in prostate cancer
Abstract
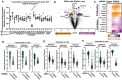
As cancer cells evade therapeutic pressure and adopt alternate lineage identities not commonly observed in the tissue of origin, they likely adopt alternate metabolic programs to support their evolving demands. Targeting these alternative metabolic programs in distinct molecular subtypes of aggressive prostate cancer may lead to new therapeutic approaches to combat treatment-resistance. We identify the poorly studied metabolic enzyme Oxoglutarate Dehydrogenase-Like (OGDHL), named for its structural similarity to the tricarboxylic acid (TCA) cycle enzyme Oxoglutarate Dehydrogenase (OGDH), as an unexpected regulator of tumor growth, treatment-induced lineage plasticity, and DNA Damage in prostate cancer. While OGDHL has been described as a tumor-suppressor in various cancers, we find that its loss impairs prostate cancer cell proliferation and tumor formation. Loss of OGDHL profoundly alters Androgen Receptor inhibition-induced plasticity, including suppressing the neuroendocrine markers DLL3 and HES6, induces accumulation of the DNA damage response marker ƔH2AX, and reduces nucleotide synthesis. Our data suggest that OGDHL has minimal impact on TCA cycle activity, and that mitochondrial localization is not required for its regulation of prostate cancer plasticity and nucleotide metabolism. Finally, we demonstrate that OGDHL expression is tightly correlated with neuroendocrine differentiation in clinical prostate cancer. These findings underscore the importance of investigating poorly characterized metabolic genes as potential regulators of distinct molecular subtypes of aggressive cancer.
Figures






References
-
- Kinnaird A., Zhao S., Wellen K. E. & Michelakis E. D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer 16, 694–707 (2016). - PubMed
Publication types
Grants and funding
- T32 GM152342/GM/NIGMS NIH HHS/United States
- TL1 DK132768/DK/NIDDK NIH HHS/United States
- U2C CA271894/CA/NCI NIH HHS/United States
- U2C DK119889/DK/NIDDK NIH HHS/United States
- R01 CA270108/CA/NCI NIH HHS/United States
- R37 CA286450/CA/NCI NIH HHS/United States
- R01 CA208642/CA/NCI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- U2C DK119886/DK/NIDDK NIH HHS/United States
- P50 CA092131/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- R01 CA267721/CA/NCI NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- OT2 OD030544/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
