This is a preprint.
A human ex vivo model of radiation-induced skin injury reveals p53-driven DNA damage signaling and recapitulates a TGFβ fibrotic response
- PMID: 40502183
- PMCID: PMC12157476
- DOI: 10.1101/2025.06.04.657901
A human ex vivo model of radiation-induced skin injury reveals p53-driven DNA damage signaling and recapitulates a TGFβ fibrotic response
Abstract
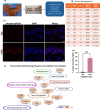
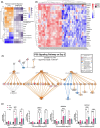
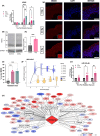
Radiation-induced skin injury is a poorly understood complication affecting cancer patients who undergo radiotherapy, with no current therapies able to prevent or halt its progression to debilitating radiation-induced skin fibrosis (RISF). Addressing the need for clinically relevant human models, this study developed and characterized a human ex vivo skin model that recapitulates the temporal molecular processes of cutaneous radiation injury, as demonstrated through bulk RNA-sequencing and tissue validation studies. Human skin explants subjected to ionizing radiation demonstrated rapid induction of DNA double-strand breaks, followed by a robust, p53-driven transcriptional program involving genes related to cell cycle arrest, apoptosis, and senescence. Over time, the irradiated skin exhibited increasing activation of pro-fibrotic pathways, notably epithelial-mesenchymal transition and TGFβ1-mediated signaling. This resulted in upregulation of classic fibrosis markers such as COL1A1, FN1, and increased collagen thickness. Importantly, regulators of the p53 axis, MDM2 and miR-34a, was observed, implicating these factors as potential therapeutic targets to modulate the balance between repair of radiation injury and pathologic fibrosis. Transcriptome analysis of irradiated and non-irradiated breast skin from post-mastectomy patients showed notable concordance of p53 and pro-fibrotic gene signatures comparable to the ex vivo model, underscoring its translational relevance. This work provides a platform for identifying early biomarkers and testing therapeutic strategies to prevent or mitigate cutaneous radiation toxicities, including RISF, beginning with elucidating the dynamic interplay between the p53-mediated DNA damage response and the onset of fibrosis following radiation. Ultimately, this work aims to improve long-term skin health and quality of life for cancer patients.
Conflict of interest statement
COMPETING INTERESTS: None
Figures


References
-
- Bentzen S. M., Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6, 702–713 (2006). - PubMed
-
- Stone H. B., Coleman C. N., Anscher M. S., McBride W. H., Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 4, 529–536 (2003). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
