Double-Masked, Dose-Response, Vehicle-Controlled Study of VVN461 Ophthalmic Solution in Postoperative Ocular Inflammation
- PMID: 40502294
- PMCID: PMC12156152
- DOI: 10.1016/j.xops.2025.100806
Double-Masked, Dose-Response, Vehicle-Controlled Study of VVN461 Ophthalmic Solution in Postoperative Ocular Inflammation
Abstract
Purpose: To evaluate the safety and ocular efficacy of VVN461, a Janus kinase kinase inhibitor, for treatment of postoperative inflammation.
Design: This was a phase II, multicenter, double-masked, randomized, vehicle-controlled, parallel-comparison study in subjects who underwent routine unilateral cataract extraction and lens replacement surgery via phacoemulsification without surgical complication.
Participants: Ninety-one subjects at 9 private practice US ophthalmology offices.
Intervention: VVN61 0.5% and 1.0% topical ophthalmic solution or vehicle, 4 times daily.
Main outcome measures: The proportion of subjects with anterior chamber cell (ACC) grade 0 in the study eye at day 14.
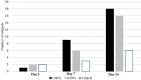
Results: The proportion of subjects with ACC grade 0 in the study eye at day 14 was 60.0% (18/30), 53.3% (16/30), and 19.4% (6/31) in the VVN461, 1.0%, VVN461, 0.5%, and vehicle groups, respectively (P = 0.0012 and 0.0057). Treatment effects in favor of VVN461 were also seen in the need for rescue medication and reduction of anterior chamber flare. There were relatively few adverse events in either VVN461 treatment group (10%, 3/30, or less), and they were judged of mild severity.
Conclusions: In this double-masked, vehicle-controlled study, clinically and statistically significant anti-inflammatory efficacy was seen with few safety issues.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Cataract surgery; Inflammation; JAK kinase; VVN461.
© 2025 by the American Academy of Ophthalmologyé.
Figures
References
-
- Robin A.L., Novack G.D., Covert D.W., et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;14:533–540. - PubMed
-
- Schwartz A. Efficacy and safety of Clobetasol propionate ophthalmic nanoemulsion, 0.05% for the treatment of intraocular inflammation and pain associated with cataract surgery. Invest Ophthalmol Vis Sci. 2023;64:3314.
LinkOut - more resources
Full Text Sources

