IL-2 immunotherapy rescues irradiation-induced T cell exhaustion in mouse colon cancer
- PMID: 40502703
- PMCID: PMC12158513
- DOI: 10.1016/j.isci.2025.112639
IL-2 immunotherapy rescues irradiation-induced T cell exhaustion in mouse colon cancer
Abstract
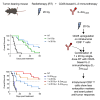
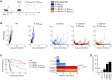
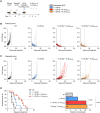
Radiotherapy (RT) can stimulate anti-cancer T cell responses, and cytokines, notably interleukin-2 (IL-2), are necessary for optimal T cell function and memory. However, timing and IL-2 receptor (IL-2R) bias of IL-2 signals are ill-defined. Using image-guided RT in a mouse colon cancer model, we observed single high-dose (20 Gy) RT transiently upregulated IL-2Rα (CD25) on effector CD8+ T cells, facilitating the use of CD25-biased IL-2 immunotherapy. Timed administration of CD25-biased IL-2 treatment after RT favored intratumoral expansion of CD8+ T cells over regulatory T cells, which resulted in comparable anti-tumor effects as with RT plus IL-2Rβ (CD122)-biased IL-2 immunotherapy. Moreover, intratumoral CD8+ T cells of animals receiving combined IL-2R-biased IL-2 and RT showed reduced markers of exhaustion. These combination treatments affected both primary irradiated and distant non-irradiated tumors and achieved durable responses. We demonstrate that timed IL-2R subunit-biased IL-2 immunotherapy synergizes with single high-dose RT to achieve potent anti-cancer immunity.
Keywords: Cancer; Immunology; Microenvironment.
© 2025 The Authors.
Conflict of interest statement
O.B. is a shareholder of Anaveon AG, developing CD122-biased IL-2 immunotherapy for cancer. O.B. and M.E.R. hold patents on improved IL-2 formulations and are shareholders of Seito Biologics AG, which develops improved IL-2 immunotherapies for autoimmune diseases. The other authors declare no competing financial interests.
Figures




References
-
- Dewan M.Z., Galloway A.E., Kawashima N., Dewyngaert J.K., Babb J.S., Formenti S.C., Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

