Cytohesin-4/ARF6 facilitates the progression of acute myeloid leukemia through activating PIK3R5/PI3K/AKT pathway
- PMID: 40502711
- PMCID: PMC12152671
- DOI: 10.1016/j.isci.2025.112634
Cytohesin-4/ARF6 facilitates the progression of acute myeloid leukemia through activating PIK3R5/PI3K/AKT pathway
Abstract
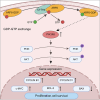
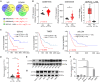
In silico analysis revealed an elevated expression of cytohesin-4 (CYTH4) in acute myeloid leukemia (AML) cells, correlating with a poorer prognosis for AML patients. However, its role in AML is not fully understood. Our study using loss-of-function assays identified CYTH4 as an oncogene promoting leukemogenesis. Silencing CYTH4 in MV4-11 and THP-1 cells reduced cell proliferation and colony formation, and induced apoptosis and cell-cycle arrest at G0/G1, whereas overexpression had no significant impact. CYTH4 silencing also increased chemosensitivity to cytarabine. In a THP-1 xenograft model, CYTH4 silencing slowed AML progression and reduced leukemic cell homing and infiltration. Mechanistically, CYTH4 silencing inhibited PI3K/AKT pathway by lowering PIK3R5 and decreased ARF6-GTP levels, as confirmed by pull-down assays. Overexpression of PIK3R5 and AKT activation via SC-79 successfully countered the cellular dysfunctions from CYTH4 silencing. Thus, CYTH4 may play a role in AML progression, and targeting its pathway could be a promising anti-leukemic treatment strategy.
Keywords: Cancer; Cell biology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Xu X., Wang J., Tong T., Zhang W., Wang J., Ma W., Wang S., Zhou D., Wu J., Jiang L., Zhao M. A self-assembled leucine polymer sensitizes leukemic stem cells to chemotherapy by inhibiting autophagy in acute myeloid leukemia. Haematologica. 2022;107:2344–2355. doi: 10.3324/haematol.2021.280290. - DOI - PMC - PubMed
-
- Khateb A., Deshpande A., Feng Y., Finlay D., Lee J.S., Lazar I., Fabre B., Li Y., Fujita Y., Zhang T., et al. The ubiquitin ligase RNF5 determines acute myeloid leukemia growth and susceptibility to histone deacetylase inhibitors. Nat. Commun. 2021;12:5397. doi: 10.1038/s41467-021-25664-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

