Organometallic Catalysis Catches up with Enzymatic in the Regeneration of NADH
- PMID: 40502976
- PMCID: PMC12150521
- DOI: 10.1021/acscatal.5c02162
Organometallic Catalysis Catches up with Enzymatic in the Regeneration of NADH
Abstract
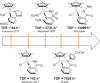
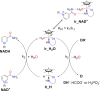
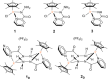
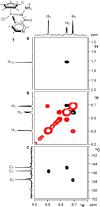
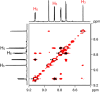
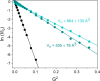
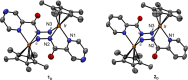
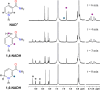
A rational design, based on a deep understanding of the reaction mechanism, led to the development of an iridium organometallic catalyst with activity comparable to that of enzymes in the chemical regeneration of NADH, using phosphite as a reducing agent. The innovative structural elements were individuated in replacing pyridine with pyrazine and adding a carbohydrazide moiety in the bidentate amidate supporting ligand. Resulting [Cp*Ir-(pyza-NH2)-Cl] (pyza-NH2 = κ2-pyrazinecarbohydrazide; 1) outperforms both the analogous complex with pyridine, [Cp*Ir-(pica-NH2)-Cl] (pica-NH2= κ2-pyridincarbohydrazide; 2), and that missing the -NH2 moiety, [Cp*Ir-(pyza)-Cl] (pyza = κ2-pyrazineamidate; 3). A maximum TOF of 13,090 h-1 was observed for 1 ([NAD+] = 6 mM, [cat] = 7.5 μM, pH 6.58 by phosphite buffer 0.4 M, 313 K), a value never reached for any organometallic catalyst and comparable with that of enzymes. 1H diffusion NMR experiments indicate that 1 and 2 undergo dimerization in water through the ionization of the Ir-Cl bond and coordination of the carbohydrazide moiety to a second iridium center. This leads to [Cp*Ir-(pyza-NH2)]2X2 (1 D ) and [Cp*Ir-(pica-NH2)]2X2 (2 D ) that were isolated as PF6 - salts by anion metathesis with NH4PF6 and fully characterized both in solution (multinuclear multidimensional NMR) and in the solid state (single-crystal X-ray diffractometry). Catalytic NADH regeneration experiments were carried out starting from stock solutions of 1-3 complexes in acetonitrile, where diffusion NMR experiments ensure the main presence of mononuclear catalytic precursors, in order to avoid complications due to dimerization. In-depth kinetic studies evidenced that catalyst 1 in combination with the H2PO3 - donor is superior to 2 and 3 in all aspects, facilitating the formation of the Ir-H intermediate and the tendency to donate the hydride to NAD+, at the same time inhibiting the detrimental accumulation of the off-cycle cat/NAD+ adduct. The introduction of the pyrazine moiety, much less σ-donating and more π-accepting than the pyridine one, is likely responsible for most of the increased activity and stability of 1 with respect to 2. Meanwhile, the dandling -NH2 carbohydrazide moiety might further accelerate the process by providing a basic functionality close to the reactive coordination position and introducing some steric hindrance to hamper the formation of the off-cycle cat/NAD+ adduct.
Keywords: NADH regeneration; NMR; X-ray diffractometry; iridium catalysts; organometallic chemistry.
© 2025 The Authors. Published by American Chemical Society.
Figures






References
-
- Das, P. ; Sen, P. . Relevance of Oxidoreductases in Cellular Metabolism and Defence. In Reactive Oxygen Species - Advances and Developments; IntechOpen, 2024. 10.5772/INTECHOPEN.112302. - DOI
-
- Ma S. K., Gruber J., Davis C., Newman L., Gray D., Wang A., Grate J., Huisman G. W., Sheldon R. A.. A Green-by-Design Biocatalytic Process for Atorvastatin Intermediate. Green Chemistry. 2010;12(1):81–86. doi: 10.1039/B919115C. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
