Fecal gut microbiota and amino acids as noninvasive diagnostic biomarkers of Pediatric inflammatory bowel disease
- PMID: 40503566
- PMCID: PMC12164387
- DOI: 10.1080/19490976.2025.2517828
Fecal gut microbiota and amino acids as noninvasive diagnostic biomarkers of Pediatric inflammatory bowel disease
Abstract
Background and aims: Fecal calprotectin (FCP) has limited specificity as diagnostic biomarker of pediatric inflammatory bowel disease (IBD), leading to unnecessary invasive endoscopies. This study aimed to develop and validate a fecal microbiota and amino acid (AA)-based diagnostic model.
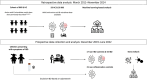
Methods: Fecal samples from a discovery cohort (de novo IBD and healthy controls [HC]) were used to develop the diagnostic model. This model was applied in a validation cohort (de novo IBD and controls with gastrointestinal symptoms [CGI]). Microbiota and AAs were analyzed using interspace profiling and liquid chromatography-mass spectrometry techniques, respectively. Machine learning techniques were used to build the diagnostic model.
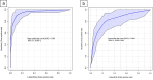
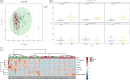
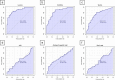
Results: In the discovery cohort (58 IBD, 59 hC), two microbial species (Escherichia coli and Alistipes finegoldii) and four AAs (leucine, ornithine, taurine, and alpha-aminoadipic acid [AAD]) combined allowed for discrimination between both subgroups (AUC 0.94, 95% CI [0.89, 0.98]). In the validation cohort (43 IBD, 38 CGI), this panel of six markers could differentiate patients with IBD from CGI with an AUC of 0.84, 95% CI [0.67, 0.95]). Leucine showed the best diagnostic performance (AUC 0.89, 95% CI [0.81, 0.95]).
Conclusions: Leucine might serve as adjuvant noninvasive biomarker in the diagnostic work-up of pediatric IBD. Future research should investigate whether the combination of leucine with FCP could improve specificity and may help tailor the course of diagnostics.
Keywords: Paediatric inflammatory bowel disease; amino acids; gut microbiota.
Conflict of interest statement
JvL reports consulting, travel and/or speaker fees and research support from AbbVie, Janssen, Nestlé Health Science, Novalac, Pfizer, Merck, P&G, GSK, Illumina, Otsuka. NdB has served as a speaker for AbbVie and MSD and has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda. This was all outside the submitted work. EV, JJ, EL, ES, AB, NVD, MB, BK, RdJ, MAB, AA, and TdM certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter of materials discussed in the manuscript.
Figures

References
-
- Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345–53.e4. doi: 10.1053/j.gastro.2019.01.002. - DOI - PubMed
-
- Ghione S, Sarter H, Fumery M, Armengol-Debeir L, Savoye G, Ley D, Spyckerelle C, Pariente B, Peyrin-Biroulet L, Turck D, et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease (1988–2011): a population-based study of French adolescents. Off J Am Coll Gastroenterol | ACG. 2018;113(2):265–272. doi: 10.1038/ajg.2017.228. - DOI - PubMed
-
- Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho K-L, Veres G, Russell RK, Paerregaard A, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795–806. doi: 10.1097/MPG.0000000000000239. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
