Influence of the pili of Lacticaseibacillus rhamnosus GG on its encapsulation and survival in mixed protein-starch gels assembled by in situ fermentation
- PMID: 40503886
- PMCID: PMC12285250
- DOI: 10.1128/aem.00248-25
Influence of the pili of Lacticaseibacillus rhamnosus GG on its encapsulation and survival in mixed protein-starch gels assembled by in situ fermentation
Abstract
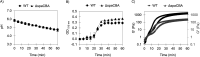
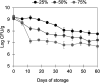
Preserving the viability of probiotics during storage and gastrointestinal digestion poses a significant challenge in the development of effective probiotic formulations. Thus, this study developed an in situ fermentation approach to encapsulate the probiotic Lacticaseibacillus rhamnosus GG (LGG) in a mixed whey protein/modified starch gel and evaluated (i) the role of the pili on gel formation and on the effectiveness of the gel to maintain cell viability during simulated digestion, and (ii) the storage stability of the encapsulated probiotics. Kinetic data of gels made with the wild-type (WT) or a pilus-depleted mutant (ΔspaCBA) strain showed a rapid in situ gel formation (<30 min) at room temperature after inoculating the polymeric mixture, driven by in situ fermentation and independently of the piliation of the cells. After simulated gastrointestinal digestion, the viability of encapsulated WT cells was ~3 log higher than free WT cells (P value 7 × 10-4) and ~0.6 times higher than encapsulated ΔspaCBA cells (P value 9 × 10-3). A higher release of ΔspaCBA vs WT cells from the gels was quantified, and confocal microscopy revealed the aggregation of ΔspaCBA but not WT cells within the gel cavities. These findings suggest the pili-dependent retention of LGG within the gel contributes to its protective effect. Finally, the hydrated gels sustained counts of LGG of 7.76-6.69 log CFU/g (depending on the relative humidity) during 2 months of storage at room temperature. In summary, bacteria-to-matrix interactions might influence the survival of probiotics during delivery, and the protein/starch gels could represent a cost-effective alternative for unrefrigerated storage and delivery of probiotics.
Importance: Many probiotic formulations struggle to maintain the viability of microbial cells over time and during their passage through the gastrointestinal tract. This has led to the development of encapsulation strategies for probiotics, most of which are either costly to implement or damage the cells during the encapsulation process. To overcome these limitations, this work developed a rapid fermentation-based approach to encapsulate probiotics in protein/starch gels as a strategy to keep the cells alive during storage and digestion. Moreover, this work explored the role of interactions between bacterial cells and their encapsulation matrix on the formation of the gels and in the protection the gels provided in maintaining the viability of cells during simulated digestion. Developing this in situ fermentation approach for the encapsulation of probiotics and understanding the bacteria-matrix interactions will lead to the development of more effective probiotic products that can be easily deployed in low-resource settings.
Keywords: encapsulation; gel; pili; probiotic; protein; starch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The potential probiotic role of Lacticaseibacillus rhamnosus on growth performance, gut health, and immune responses of weaned pigs.J Anim Sci. 2025 Jan 4;103:skaf089. doi: 10.1093/jas/skaf089. J Anim Sci. 2025. PMID: 40125886
-
Development and Characterization of Synbiotic Microbeads for Enhanced Viability of Probiotics for Food Applications.J Food Sci. 2025 Jul;90(7):e70390. doi: 10.1111/1750-3841.70390. J Food Sci. 2025. PMID: 40610194
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
In vitro controlled release of the probiotic strain Bacillus licheniformis PPL2016 microencapsulated: Simulating the digestive system by age class and sex in the blue swimming crab Callinectes arcuatus.Braz J Microbiol. 2025 Sep;56(3):2007-2026. doi: 10.1007/s42770-025-01674-1. Epub 2025 May 4. Braz J Microbiol. 2025. PMID: 40319424
-
Probiotics for the prevention of pediatric antibiotic-associated diarrhea.Cochrane Database Syst Rev. 2011 Nov 9;(11):CD004827. doi: 10.1002/14651858.CD004827.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Dec 22;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. PMID: 22071814 Updated.
References
-
- Levels and trends in child malnutrition: UNICEF/WHO/World Bank Group joint child malnutrition estimates: key findings of the 2023 edition. Available from: https://www.who.int/publications/i/item/9789240073791. Retrieved 06 Nov 2024.
-
- Castro-Mejía JL, O’Ferrall S, Krych Ł, O’Mahony E, Namusoke H, Lanyero B, Kot W, Nabukeera-Barungi N, Michaelsen KF, Mølgaard C, Friis H, Grenov B, Nielsen DS. 2020. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 11:855–867. doi: 10.1080/19490976.2020.1712982 - DOI - PMC - PubMed
-
- Kambale RM, Ntagazibwa JN, Kasengi JB, Zigashane AB, Francisca IN, Mashukano BN, Amani Ngaboyeka G, Bahizire E, Zech F, Bindels LB, Van der Linden D. 2023. Probiotics for children with uncomplicated severe acute malnutrition (PruSAM study): a randomized controlled trial in the Democratic Republic of Congo. Am J Clin Nutr 117:976–984. doi: 10.1016/j.ajcnut.2023.01.019 - DOI - PubMed
-
- Grenov B, Namusoke H, Lanyero B, Nabukeera-Barungi N, Ritz C, Mølgaard C, Friis H, Michaelsen KF. 2017. Effect of probiotics on diarrhea in children with severe acute malnutrition: a randomized controlled study in Uganda. J Pediatr Gastroenterol Nutr 64:396–403. doi: 10.1097/MPG.0000000000001515 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

