Arsenic Trioxide Enhances the Efficacy of PD-1 Inhibitors in Hepatocellular Carcinoma by Inducing Immunogenic Cell Death via the ROS/ERS Pathway
- PMID: 40504080
- PMCID: PMC12160338
- DOI: 10.1002/iid3.70214
Arsenic Trioxide Enhances the Efficacy of PD-1 Inhibitors in Hepatocellular Carcinoma by Inducing Immunogenic Cell Death via the ROS/ERS Pathway
Abstract
Background: Hepatocellular carcinoma (HCC) remains a major global health challenge, with limited efficacy of current immunotherapeutic strategies. Immunogenic cell death (ICD), characterized by the release of damage-associated molecular patterns (DAMPs), offers a promising approach to enhance antitumor immunity. Arsenic trioxide (ATO), an ICD inducer, may synergize with PD-1 inhibitors to overcome therapeutic resistance, though the underlying mechanisms remain unclear.
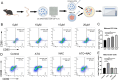
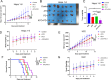
Methods: The cytotoxicity of ATO was evaluated via MTT, clonogenic, and apoptosis assays. ROS levels were quantified using ROS fluorescent probes. ERS activation was confirmed by Western blot detection of Calnexin, PDI, ATF-4, p-elF2α, and Caspase-12. ICD induction was assessed by measuring DAMPs (CRT exposure, HMGB1/ATP/IFN-β release). The roles of ROS/ERS pathways were dissected using NAC (ROS inhibitor) or 4-PBA (ERS inhibitor) pre-treatment. Ex vivo dendritic cell maturation assays analyzed ATO-treated HCC cells' immunostimulatory capacity, while In Vivo models evaluated immune microenvironment modulation via flow cytometry. Prophylactic/therapeutic tumor vaccine experiments assessed antitumor immunity using ATO-treated HCC cells as vaccines. Synergy between ATO and PD-1 blockade was tested in tumor-bearing mice by combining ATO with anti-PD-1 antibodies, monitoring tumor growth kinetics and survival outcomes.
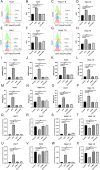
Results: ATO dose-dependently reduced HCC cell viability while elevating intracellular ROS levels and activating ERS. These processes triggered the release/surface exposure of ICD-related DAMPs, including CRT, HMGB1, ATP, and IFN-β, leading to dendritic cells maturation and tumor immune microenvironment remodeling. ATO-treated HCC cells exhibited enhanced immunogenicity, functioning as prophylactic and therapeutic vaccines to stimulate antitumor immunity. Notably, ATO significantly potentiated the therapeutic efficacy of PD-1 inhibitors In Vivo.
Conclusion: ATO induces ICD in HCC via a ROS/ERS signaling axis, thereby amplifying antitumor immune responses and synergizing with PD-1 blockade. These findings support the clinical evaluation of ATO-PD-1 inhibitor combinations to improve outcomes in HCC patients.
Keywords: arsenic trioxide; endoplasmic reticulum stress; hepatocellular carcinoma; immunogenic cell death; oxidative stress.
© 2025 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Arsenic trioxide augments immunogenic cell death and induces cGAS-STING-IFN pathway activation in hepatocellular carcinoma.Cell Death Dis. 2024 Apr 29;15(4):300. doi: 10.1038/s41419-024-06685-8. Cell Death Dis. 2024. PMID: 38684648 Free PMC article.
-
Piperlongumine enhances the antitumor efficacy of PD-1 inhibitors by inducing immunogenic cell death in prostate cancer cells.World J Urol. 2025 Jul 2;43(1):406. doi: 10.1007/s00345-025-05760-9. World J Urol. 2025. PMID: 40601121
-
Liensinine reshapes the immune microenvironment and enhances immunotherapy by reprogramming metabolism through the AMPK-HIF-1α axis in hepatocellular carcinoma.J Exp Clin Cancer Res. 2025 Jul 15;44(1):208. doi: 10.1186/s13046-025-03477-6. J Exp Clin Cancer Res. 2025. PMID: 40665352 Free PMC article.
-
Immunological Landscape and Molecular Therapeutic Targets of the Tumor Microenvironment in Hepatocellular Carcinoma.Int J Mol Sci. 2025 Aug 13;26(16):7836. doi: 10.3390/ijms26167836. Int J Mol Sci. 2025. PMID: 40869156 Free PMC article. Review.
-
Multidimensional insights into exosomes in hepatocellular carcinoma: from genesis to clinical application.Front Immunol. 2025 Aug 13;16:1628573. doi: 10.3389/fimmu.2025.1628573. eCollection 2025. Front Immunol. 2025. PMID: 40881702 Free PMC article. Review.
References
-
- Vogel A., Meyer T., Sapisochin G., Salem R., and Saborowski A., “Hepatocellular Carcinoma,” The Lancet 400, no. 10360 (2022): 1345–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

