Improving shared decision‑making between paediatric haematologists, children with sickle cell disease, and their parents: an observational post-intervention study
- PMID: 40504306
- PMCID: PMC12162739
- DOI: 10.1007/s00431-025-06241-2
Improving shared decision‑making between paediatric haematologists, children with sickle cell disease, and their parents: an observational post-intervention study
Abstract
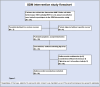
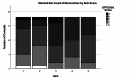
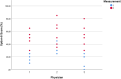
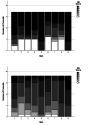
Children with sickle cell disease (SCD) suffer from a chronic disease that can lead to serious co-morbidity and impacts their quality of life. During the course of their disease, a variety of health-related decisions need to be made for and by SCD-patients, depending on their age and health status, together with their parents and paediatric haematology clinicians. Shared decision-making (SDM) may improve health outcomes of chronically ill children but is still not commonly applied. We assessed the level of SDM among paediatric haematologists after the introduction of SDM interventions. An observational post-intervention study was conducted in a paediatric outpatient clinic of a university hospital. After an SDM consultation training of the three paediatric haematologists and introduction of SDM-supporting tools for both paediatricians and (parents of) patients with SCD, two evaluators independently and objectively analysed the level of patient involvement in decision-making from audio-recordings of the consultations using the OPTION-5 instrument. SDM-Q-9 and SDM-Q-Doc questionnaires were used to measure the level of SDM as perceived by patients/parents and paediatricians, respectively. Scores were expressed as a percentage, ranging from 0% (no SDM observed) to 100% (exemplary level of SDM). Participants were 9 female and 9 male patients between 4 months and 17 years old, with a median age of 7.5 years (Interquartile Range [IQR] 2.5-12). Eighteen consultations (six per paediatrician) in which a decision was to be made about SCD treatment options were analysed. Median OPTION-5 score was 50 (IQR 40-65%). Median SDM-Q-9 and SDM-Q-Doc scores were 73% (IQR 52.2-91) and 62.2% (IQR 55.6-71.1), respectively.
Conclusion: After the introduction of SDM training and tools, paediatric haematologists reached a moderately good level of SDM. This level had doubled as compared to the baseline level, as assessed in a previous study.
What is known: • Children who suffer from sickle cell disease (SCD) are vulnerable to health inequities and suboptimal health outcomes. Hence, SDM seems an appropriate method of care for these children. • SDM tools and training may help paediatricians and children participate in a collaborative decision-making process about the children's preferred treatment options and improve their health outcomes.
What is new: • After SDM training and decision support aids for paediatricians and patients, the level of involvement in the decision-making process by (the parents of) patients suffering from SCD reached a moderately good level. • A difference persists between paediatricians' perceived level of involving the child and parents in a shared decision-making process and the observed level of involvement.
Keywords: Chronic disease; OPTION-instrument; Paediatrics; SDM-Q-9 questionnaire; SDM-Q-Doc questionnaire; Shared decision-making; Sickle cell disease; Triadic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Under Amsterdam UMC Ethics board registration number W21_312 # 21.347, informed consent was obtained from all the participants according to Dutch law and guidelines in accordance with the Declaration of Helsinki as follows: Parents of children younger than 12 years provided written informed consent. Children between 12 and 16 years co-signed the informed consent form together with their parents. Children 16 years and older provided written informed consent on their own. Consent for publication: N/A. Conflict of interests: The authors declare no competing interests.
Figures

References
-
- Wyatt KD et al (2015) Shared decision making in pediatrics: a systematic review and meta-analysis. Acad Pediatr 15(6):573–583 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

