Sustained Effectiveness, Tolerability, and Safety of Long-Term Prophylaxis with Lanadelumab in Hereditary Angioedema: The Prospective, Phase 4, Noninterventional EMPOWER Real-World Study
- PMID: 40504359
- PMCID: PMC12313730
- DOI: 10.1007/s12325-025-03226-3
Sustained Effectiveness, Tolerability, and Safety of Long-Term Prophylaxis with Lanadelumab in Hereditary Angioedema: The Prospective, Phase 4, Noninterventional EMPOWER Real-World Study
Abstract
Introduction: Lanadelumab is approved for long-term prophylaxis of hereditary angioedema (HAE) attacks in patients aged ≥ 2 years in the USA and aged ≥ 12 years in Canada. The EMPOWER Study (NCT03845400) evaluated the real-world effectiveness and safety of lanadelumab in male and female patients with HAE due to C1 inhibitor deficiency type 1 or 2 from the USA and Canada. Here, we report final, up to 36-month, data.
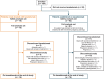
Methods: Patients aged ≥ 12 years were classified as newly treated with lanadelumab or established on lanadelumab (receiving < 4 and ≥ 4 lanadelumab doses at enrollment, respectively). The primary objective was effectiveness of lanadelumab as measured by HAE attack rate before and after lanadelumab initiation. Safety data were collected.
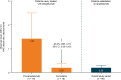
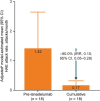
Results: A total of 109 patients received ≥ 1 lanadelumab dose and had ≥ 1 post-baseline safety assessment. Patients were 40.9 (17.4) years of age (mean [standard deviation (SD)]), majority (72/109; 66.1%) female, 37/109 (33.9%) male, and over 90% white. Patients newly treated with and established on lanadelumab received lanadelumab for 737.7 (374.5) (mean [SD]) and 907.1 (469.3) days, respectively, during the study. In patients newly treated with lanadelumab, the mean (95% confidence interval) observed attack rate (attacks/month) decreased by 85% after lanadelumab initiation, from 1.42 (0.34-2.50) pre-lanadelumab to 0.20 (0.02-0.38) post-lanadelumab initiation (cumulative period). Patients established on lanadelumab had an observed attack rate of 0.20 (0.10-0.30) during 36 months' follow-up. Of 154 treatment-emergent adverse events (TEAEs), no injection site reactions were reported and 6 (in 2 patients) were considered related to lanadelumab; no lanadelumab-related TEAEs were serious.
Conclusion: Real-world data from EMPOWER showed marked HAE attack rate reduction up to 36 months after initiating lanadelumab in patients newly treated with lanadelumab and maintenance of low attack rates in patients established on lanadelumab. No new safety signals were identified.
Trial registration: ClinicalTrials.gov, identifier NCT03845400. Graphical abstract available for this article.
Keywords: Effectiveness; Hereditary angioedema; Lanadelumab; Long-term prophylaxis; Real-world data; Safety.
© 2025. Takeda Development Center Americas Inc.
Conflict of interest statement
Declarations. Conflict of Interest: Jonathan A. Bernstein has been or is currently a clinical investigator for BioCryst, BioMarin Pharmaceutical, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharming Group, and Takeda; consultant for BioCryst, BioMarin Pharmaceutical, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharming Group, and Takeda; and a member of the Hereditary Angioedema Medical Advisory Board and US Hereditary Angioedema Association. Stephen D. Betschel has been a speaker and/or received advisor fees from Astria Therapeutics, BioCryst, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharvaris, and Takeda; and research funding from CSL Behring and Takeda. Paula J. Busse has received research support and served on advisory boards for ADARx, Astria Therapeutics, BioCryst, CSL Behring, Intellia Therapeutics, KalVista Pharmaceuticals, and Takeda; and is a consultant for CVS Pharmacy. Aleena Banerji has received research support from Astria Therapeutics, Ionis, and Takeda; and been an advisory board member for ADARx Pharmaceuticals, Astria Therapeutics, BioCryst, BioMarin Pharmaceutical, CSL Behring, Intellia Therapeutics, KalVista Pharmaceuticals, Pharvaris, and Takeda. H. James Wedner has received research support from Astria Therapeutics, BioCryst, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharming Group, Pharvaris, and Takeda; been a speaker for BioCryst, BioMarin Pharmaceutical, Blueprint Medicines, CSL Behring, KalVista Pharmaceuticals, Pharming Group, and Takeda; and consulted for ADARx Pharmaceuticals, Astria Therapeutics, BioCryst, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharming Group, and Takeda. Michael Manning has been a researcher/primary investigator and speaker/consultant for BioCryst, BioMarin Pharmaceutical, CSL Behring, Ionis, KalVista Pharmaceuticals, Pharming Group, Pharvaris, and Takeda. Rafael H. Zaragoza-Urdaz has been or is currently a clinical investigator for KalVista Pharmaceuticals, Pharvaris, and Takeda; has received honoraria from/been a member of a speaker’s bureau for BioCryst, CSL Behring, Pharming Group, and Takeda; been an advisory board member for BioCryst, Pharming Group, Regeneron, and Takeda; and received research support from AstraZeneca, GlaxoSmithKline, KalVista Pharmaceuticals, and Takeda. John Anderson has received personal fees for steering committee, advisory board, speaker bureau, and/or clinical research activity from BioCryst, BioMarin Pharmaceutical, CSL Behring, Cycle Pharmaceuticals, KabiCare, KalVista Pharmaceuticals, Pharming Group, Pharvaris, and Shire/Takeda. Remi Gagnon has served as a researcher for ALK-Abelló, AstraZeneca, DBV Technologies, GlaxoSmithKline, Moderna, Novartis, Regeneron, Sanofi, and Takeda; been an advisory board member for ALK-Abelló, Bausch & Lomb, Regeneron, Sanofi Genzyme, and Takeda; and received fees for speaker bureaus from Bausch & Lomb and Novartis. Alan P. Baptist reports research support from BioCryst, Ionis, and Takeda. Daniel Soteres is an advisor for CSL Behring, Cycle Pharmaceuticals, KalVista Pharmaceuticals, and Shire/Takeda; consultant for BioCryst, KalVista Pharmaceuticals, and Pharming Group; conducted research for BioCryst, BioMarin Pharmaceutical, Ionis, KalVista Pharmaceuticals, Pharvaris, and Shire/Takeda; and a speaker for BioCryst, CSL Behring, Pharming Group, and Shire/Takeda. William R. Lumry is a member of advisory boards for Astria Therapeutics, BioCryst, BioMarin Pharmaceutical, CSL Behring, Intellia Therapeutics, KalVista Pharmaceuticals, Pharvaris, and Takeda; received research grants from BioCryst, BioMarin Pharmaceutical, CSL Behring, Ionis, KalVista Pharmaceuticals, and Takeda; consulting fees from Astria Therapeutics, BioCryst, CSL Behring, Fresenius Kabi, Pharming Group, and Takeda; payments for lectures from CSL Behring, Pharming Group, and Takeda; and is an advisory board member of the US Hereditary Angioedema Association. Timothy Craig has served as a speaker and researcher for Astria Therapeutics, BioMarin Pharmaceutical, Ionis, CSL Behring, Intellia Therapeutics, KalVista Pharmaceuticals, and Takeda; researcher for Pharvaris; speaker for Grifols; consultant for Astria Therapeutics, BioCryst, BioMarin Pharmaceutical, CSL Behring, Intellia Therapeutics, Ionis, and Takeda; Director for ACARE International Hereditary Angioedema Center; and member of the Medical Advisory Board for the HAE-A. Timothy Craig is an Editorial Board member of Advances in Therapy. Timothy Craig was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Daniel Petroni reports advisory boards for Takeda and has been a research/primary investigator for BioCryst and Takeda; he is now an employee of BioCryst. F. Ida Hsu received research support from Takeda; served on advisory boards for BioCryst, CSL Behring, KalVista Pharmaceuticals, and Takeda; was a speaker for Amgen, Pharming, and Shire/Takeda; and is a consultant for Intellia Therapeutics. Daniel Nova Estepan and Maureen Watt are employees of Takeda Development Center Americas, Inc. and hold stock and/or share options in Takeda Pharmaceutical Company Limited. Salomé Juethner is an employee of Takeda Pharmaceuticals USA, Inc., and holds stock and/or share options in Takeda Pharmaceutical Company Limited. Natalie Khutoryansky is an employee of Cytel, contracted by Takeda Development Center Americas, Inc. Bruce L. Zuraw has received consulting fees from Adverum Biotechnologies, BioCryst, CSL Behring, and Takeda. Ethical Approval: EMPOWER was conducted in accordance with the International Council for Harmonisation Good Clinical Practice Guidelines, ethical principles that have their origins in the Declaration of Helsinki, and other local ethical and legal requirements. The study was reviewed and approved in all study sites by a central review board (Western-Copernicus Group [WCG] Institutional Review Board) or institutions’ local ethics committees (Table S1 in the Supplementary Material). All patients or their legally authorized representatives provided informed consent to participate in the study.
Figures

References
-
- Christiansen SC, Wilmot J, Castaldo AJ, Zuraw BL. The US Hereditary Angioedema Association Scientific Registry: hereditary angioedema demographics, disease severity, and comorbidities. Ann Allergy Asthma Immunol. 2023;131:766–74.e8. - PubMed
-
- Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24:S292–8. - PubMed
-
- Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77:1961–90. - PubMed
-
- Busse PJ, Christiansen SC, Riedl MA, et al. US HAEA Medical Advisory Board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9:132–50.e3. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

