High-dose romiplostim for donor-type aplasia
- PMID: 40504447
- PMCID: PMC12334513
- DOI: 10.1007/s00277-025-06448-1
High-dose romiplostim for donor-type aplasia
Abstract
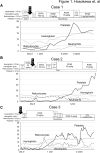
Donor-type aplasia (DTA) is a severe complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), characterized by bone marrow hypoplasia despite full chimerism. This report highlights the successful use of romiplostim (ROMI) for treating DTA in three patients with acquired aplastic anemia (AA), including two who were unresponsive to eltrombopag (EPAG). Case 1: A 21-year-old female with non-severe AA, treated with cyclosporine (CsA), rabbit antithymocyte globulin, and EPAG, showed no improvement. After undergoing bone marrow transplantation (BMT) from an HLA-matched sibling, she continued to experience pancytopenia. Switching from EPAG to ROMI 16 months after BMT led to transfusion independence after 7 weeks and normalized blood counts by 17 months. Case 2: A 35-year-old male with moderate AA, initially treated with CsA and ROMI, switched to EPAG before BMT from an HLA-matched sibling. Despite complete donor chimerism, pancytopenia recurred 7 months after BMT. Transitioning from EPAG to ROMI resulted in transfusion independence after 5 months and normalized blood counts after 11 months of ROMI. Case 3: A 25-year-old female with moderate AA, unresponsive to CsA and EPAG, remained transfusion-dependent. Following BMT from an HLA-matched sibling and an initial lack of response to ROMI, restarting ROMI led to transfusion independence after 9 months and normalization of blood counts by day 418 post-BMT. These patients suggest ROMI, particularly at high doses, may be more effective than EPAG for DTA, potentially avoiding the need for a second allo-HSCT. Further studies are required to compare the efficacy of ROMI and EPAG in treating DTA following allo-HSCT.
Keywords: Allo-BMT; Donor-type aplasia; Romiplostim.
Conflict of interest statement
Declarations. Ethical approval: The study protocol was approved by the institutional review board of Kanazawa University Hospital. Informed consent: Informed consent was obtained from the patient for publication of this report. Competing interests: Prof. Nakao and Yamazaki reports honoraria from Kyowa-Kirin and Novartis. The other authors declare no conflicts of interest in association with the present study.
Figures
References
-
- Yoshida N, Yagasaki H, Yabe H, Kikuchi A, Kobayashi R, Takahashi Y et al (2012) Donor-type aplasia after bone marrow transplantation in children with aplastic anemia: a nationwide retrospective study. Blood 120(21):959. 10.1182/blood.V120.21.959.959 - DOI
-
- Maruyama K, Aotsuka N, Kumano Y, Sato N, Kawashima N, Onda Y et al (2018) Immune-mediated hematopoietic failure after allogeneic hematopoietic stem cell transplantation: a common cause of late graft failure in patients with complete donor chimerism. Biol Blood Marrow Transplant 24(1):43–49. 10.1016/j.bbmt.2017.08.018 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

