Safety and efficacy of apixaban thrombosis prevention in pediatric patients with obesity and acute lymphoblastic leukemia
- PMID: 40505060
- PMCID: PMC12496241
- DOI: 10.1182/bloodadvances.2025016160
Safety and efficacy of apixaban thrombosis prevention in pediatric patients with obesity and acute lymphoblastic leukemia
Abstract
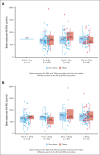
Pediatric patients with acute lymphoblastic leukemia and lymphoma (ALL/LL) and obesity are at increased risk for venous thromboembolism (VTE). The PREVAPIX-ALL trial was an open-label, randomized, controlled trial assessing the safety and efficacy of apixaban for VTE prevention in pediatric patients with ALL/LL. An a priori subgroup analysis of patients with obesity in the PREVAPIX-ALL trial was planned because of increased VTE risk in this group. Patients with obesity, aged ≥2 to <18 years, central venous catheter, and chemotherapy containing asparaginase were randomized to apixaban (prophylactic dose) vs standard of care (SOC; no anticoagulation) during induction chemotherapy. The primary efficacy end point was a composite of nonfatal symptomatic and asymptomatic VTE and VTE-related death. The primary and secondary safety outcomes were major bleeding and a composite of major and clinically relevant nonmajor (CRNM) bleeding, respectively. A total of 82 PREVAPIX-ALL participants presented with obesity, of whom 42 were randomized to apixaban. For the primary efficacy end point, a significant decrease in VTE events was present in the apixaban arm (1/42 [2.4%]) as compared with the SOC arm (10/40 [25%]; relative risk [RR], 0.09; 95% confidence interval [CI], 0.01-0.97; P = .007). There was a statistically significant treatment obesity interaction, P = .03. No statistically significant difference was observed for the primary efficacy end point among the nonobese group (RR, 0.85; 95% CI, 0.53-1.37; P = .50). No statistically significant difference in major or CRNM bleeding was observed. Apixaban prophylaxis in patients with obesity and ALL/LL resulted in a statistically significant VTE risk reduction with no increase bleeding. This trial was registered at www.clinicaltrials.gov as #NCT02369653.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.M., J.L.D., and N.A.F. are employees of Bristol Myers Squibb (BMS). The institutions of V.R. and S.H.O. received salary support from the Children’s Oncology Group for their roles as study chair and study vice-chair, respectively. E.O. served as a consultant for Jazz Pharmaceuticals; and received consulting fees from Seagen, Inc. L.G.M. received research funding from BMS for the biomarker substudy. The remaining authors declare no competing financial interests.
A complete list of the PREVAPIX-ALL investigators appears in “Appendix.”
Figures

Comment in
-
Weighing the evidence: apixaban for pediatric leukemia.Blood Adv. 2025 Sep 23;9(18):4748-4749. doi: 10.1182/bloodadvances.2025016948. Blood Adv. 2025. PMID: 40986308 Free PMC article. No abstract available.
References
-
- Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia: part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111(3):125–131. - PubMed
-
- Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. - PubMed
-
- Levy-Mendelovich S, Barg AA, Kenet G. Thrombosis in pediatric patients with leukemia. Thromb Res. 2018;164(suppl 1):S94–S97. - PubMed
-
- Nowak-Göttl U, Kenet G, Mitchell LG. Thrombosis in childhood acute lymphoblastic leukaemia: epidemiology, aetiology, diagnosis, prevention and treatment. Best Pract Res Clin Haematol. 2009;22(1):103–114. - PubMed

