Evaluation of 134Ce/134La-PSMA-617 for PET Imaging and Auger Electron Therapy of Prostate Cancer
- PMID: 40506238
- PMCID: PMC12320565
- DOI: 10.2967/jnumed.125.269751
Evaluation of 134Ce/134La-PSMA-617 for PET Imaging and Auger Electron Therapy of Prostate Cancer
Abstract
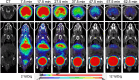
The 134Ce/134La radionuclide pair has been proposed as a PET imaging surrogate for targeted α-radiotherapeutics. 134Ce decays to 134La via electron capture, emitting Auger electrons (AEs), which could be used for targeted radionuclide therapy. Additionally, the positron emission during this transition enables PET imaging, making 134Ce/134La a promising theranostic pair for prostate cancer. In this work, we investigated the potential of 134Ce for AE capture therapy using prostate-specific membrane antigen (PSMA)-617 for targeted radionuclide delivery. Methods: Radiolabeling of [134Ce]Ce-PSMA-617 proceeded as previously described, and C18 cartridge purification was optimized. In vitro, cell-binding and toxicity assays were performed on PSMA-positive PC3 PIP cells. In vivo PET imaging and ex vivo biodistribution studies were conducted on mice bearing dual PSMA-positive PC3 PIP and PSMA-negative PC3 flu tumor xenografts at various time points ranging from 1 to 72 h after injection. Additionally, an in vivo single-dose-treatment study was performed using 37- and 111-MBq doses in nude mice with PC3 PIP tumor xenografts. Results: PSMA-617 was successfully radiolabeled with 134Ce/134La and purified using the C18 cartridge method, achieving high molar activity (21.02 ± 0.11 MBq/nmol). Stability studies showed more than 95% stability in mouse serum at day 5. PSMA-positive PC3 PIP cells demonstrated 89.6% ± 0.55% cell binding, 55.45% ± 0.96% internalization at 24 h, and a dissociation constant of 32.9 ± 3.9 nM, comparable to other reported [177Lu]Lu/[225Ac]Ac-PSMA-617 radiocomplexes. In contrast, no cellular uptake or internalization was observed in PSMA-negative PC3 flu cells. Clonogenic assay of [134Ce]Ce-PSMA-617 showed a significant dose-dependent reduction in cell proliferation (P = 0.002). PET imaging revealed high tumor-specific uptake at early time points (1 and 4 h), followed by a gradual decline from 24 to 72 h, with rapid clearance from normal tissues. These results were corroborated by ex vivo biodistribution studies. In vivo therapy with [134Ce]Ce-PSMA-617 in tumor-bearing mice demonstrated a significant increase in median survival compared with control animals (saline, 33 d; 37 MBq, 50 d; and 111 MBq, 80 d, end of the study). Conclusion: [134Ce]Ce-PSMA-617 exhibited excellent in vitro and in vivo characteristics, providing significant survival benefits in mice. Collectively, these findings suggest that [134Ce]Ce-PSMA-617 is an effective theranostic agent for PET imaging and AE therapy of prostate cancer.
Keywords: Auger electrons; [134Ce]Ce-PSMA-617; prostate cancer; targeted radiopharmaceutical therapy.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures






References
-
- Goldsmith SJ. Targeted radionuclide therapy: a historical and personal review. Semin Nucl Med. 2020;50:87–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
