Diagnostic efficacy of fecal-based miR-92a for advanced colorectal neoplasia: a prospective multicenter screening trial
- PMID: 40506768
- PMCID: PMC12164163
- DOI: 10.1186/s40779-025-00613-3
Diagnostic efficacy of fecal-based miR-92a for advanced colorectal neoplasia: a prospective multicenter screening trial
Abstract
Background: More efficacious, noninvasive screening methods are needed for advanced colorectal neoplasia. miR-92a is a reliable and reproducible biomarker for early colorectal cancer detection in stool samples. We compared the diagnostic efficacies of miR-92a, immunochemical fecal occult blood testing (FIT), and their combination (FIT + miR-92a) in a prospective multicenter screening trial.
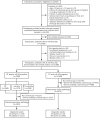
Methods: Overall, 16,240 participants aged 30-75 years were enrolled between April 1, 2021, and December 31, 2023. A total of 15,586 participants returned samples available for both FIT and miR-92a tests. All those with positive, and a random selection of those with negative screening tests were recommended to undergo colonoscopy. Follow-ups were performed until participants completed the colonoscopic examination. A total of 1401 screen-positive and 2079 randomly selected screen-negative individuals completed colonoscopies. Primary outcomes included sensitivity, number needed to screen (NNS), Youden index and receiver operating characteristic area under the curve (AUC) for advanced adenomas and colorectal cancer [advanced neoplasia (AN)] for each screening modality in the diagnostic performance analysis.
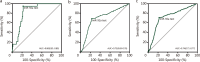
Results: Colonoscopy was performed in 3480 individuals. The colonoscopy compliance rate was 47.8% for screen-positive individuals. The sensitivity of miR-92a versus FIT for AN was 70.9% versus 54.3% (P < 0.001), NNS was 24.7 versus 32.2 (P = 0.001), Youden index was 47.9% versus 35.0% (P < 0.001), AUC was 0.74 versus 0.67 (P = 0.010). FIT + miR-92a had a sensitivity of 85.4%, an NNS of 20.5, a Youden index of 47.9% and an AUC of 0.74 for AN.
Conclusions: For AN screening, miR-92a demonstrated better sensitivity, NNS, Youden index and AUC as compared with FIT. Compared with FIT, using miR-92a appears to be more efficient for population-based screening programs. Screening sensitivity for AN can be further enhanced if conditionally used in combination with FIT.
Trial registration: Chinese Clinical Trial Registration Number: ChiCTR2200065415.
Keywords: Advanced neoplasia; Colorectal cancer; Immunochemical fecal occult blood testing; MiR-92a; Screening.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical reviews were conducted separately by the ethics committees of each clinical center [Ethics review committee of Shenzhen People’s Hospital (SYL-202123-03); Ethics review committee of Shenzhen Bao’an District Hospital of Traditional Chinese Medicine (SJ-2021-001-04); Ethics review committee of Tianjin Nankai Hospital (NKYY_YX_IRB_2021_002)], with final approval from the group leader at Shenzhen People’s Hospital. Informed consent was obtained from all eligible participants. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

