Implementation of ultra-hypofractionated radiotherapy for breast cancer in the Netherlands in 2020-2023, using registry data and questionnaires
- PMID: 40506770
- PMCID: PMC12164123
- DOI: 10.1186/s13014-025-02669-w
Implementation of ultra-hypofractionated radiotherapy for breast cancer in the Netherlands in 2020-2023, using registry data and questionnaires
Abstract
Background: This study investigated the implementation of ultra-hypofractionated radiotherapy (i.e. 5 fractions) in DCIS and early-stage breast cancer, factors associated with its use, and variation across radiotherapy institutes.
Methods: Registry and questionnaire data were used. Registry data included data from the Netherlands Cancer Registry and the NABON Breast Cancer Audit-Radiotherapy (NBCA-R). Women eligible for 5 fractions were included. Trends and variation were visualised using trendlines and case-mix adjusted boxplots. Logistic regression was applied to investigate which factors were associated with the use of 5 fractions. In April 2024 a questionnaire was distributed among radiotherapy institutes to identify facilitators and barriers for implementation.
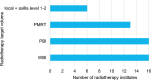
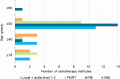
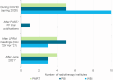
Results: The current study included 16,115 women. In 2020, 18.5% of the eligible women received 5 fractions, compared to 60.8% in 2023. The lowest variation between radiotherapy institutes was found in 2023 (median: 60.4%, interquartile range: 53.3-70.6%). Age, tumour grade, multifocality, (y)pT, (y)pN, radiotherapy target volume, type of radiotherapy institute, and start year of radiation were associated with the chance of receiving 5 fractions. Sixteen out of the 19 radiotherapy institutes completed the questionnaire, showing variation in age and radiotherapy target volume for which the schedule was used. Most institutes mentioned no barriers for using 5 fractions. Questionnaire data confirmed the trendline finding that national consensus meetings were essential for largescale implementation.
Conclusions: The use of ultra-hypofractionated radiotherapy has increased during the past four years, with reduced variation across Dutch institutes. Registry and questionnaire data indicated that national consensus meetings were instrumental in driving implementation.
Keywords: Breast cancer; Questionnaires; Radiotherapy; Registries.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Privacy Review Board of the NCR confirmed that no ethical approval was required for the current study, because of the retrospective design and the anonymized patient data. Competing Interests: The authors declare no competing interests.
Figures



References
-
- Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, Alhasso A, Barrett-Lee P, Bliss P, Bloomfield D, Bowen J. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38:3261–72. 10.1200/JCO.19.02750. - PMC - PubMed
-
- Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, Chan C, Churn M, Cleator S, Coles CE, Goodman A, Harnett A, Hopwood P, Kirby AM, Kirwan CC, Morris C, Nabi Z, Sawyer E, Somaiah N, Stones L, Syndikus I, Bliss JM, Yarnold JR, Alhasso A, Armstrong A, Bliss J, Bloomfield D, Bowen J, Brunt M, Chan C, Chantler H, Churn M, Cleator S, Coles C, Donovan E, Goodman A, Griffin S, Haviland J, Hopwood P, Kirby A, Kirk J, Kirwan C, MacLennan M, Morris C, Nabi Z, Sawyer E, Sculphur M, Sinclair J, Somaiah N, Stones L, Sydenham M, Syndikus I, Tremlett J, Venables K, Wheatley D, Yarnold J. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–26. 10.1016/S0140-6736(20)30932-6. - PMC - PubMed
-
- Coles C, Aristei C, Bliss J, Boersma L, Brunt A, Chatterjee S, Hanna G, Jagsi R, Person OK, Kirby A, Mjaaland I, Meattini I, Luis AM, Marta GN, Offersen B, Poortmans P, Rivera S. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32:279–81. 10.1016/j.clon.2020.03.006. - PMC - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), McGale P, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35. 10.1016/S0140-6736(14)60488-8. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

