A Narrative Review of Artificial Intelligence in MRI-Guided Prostate Cancer Diagnosis: Addressing Key Challenges
- PMID: 40506914
- PMCID: PMC12154491
- DOI: 10.3390/diagnostics15111342
A Narrative Review of Artificial Intelligence in MRI-Guided Prostate Cancer Diagnosis: Addressing Key Challenges
Abstract
Background/Objectives: Magnetic resonance imaging (MRI) is crucial in detecting suspicious lesions and diagnosing clinically significant prostate cancer (csPCa). However, variability in MRI-targeted diagnostic pathways arises due to factors such as patient characteristics, imaging protocols, and radiologist expertise. Artificial intelligence (AI) offers potential solutions to these challenges by enhancing diagnostic accuracy and efficiency. Methods: This narrative review explores AI techniques, particularly machine learning and deep learning, in the context of prostate cancer diagnosis. It examines their application in improving MRI scan quality, detecting artifacts, and assisting radiologists in lesion detection and interpretation. It also considers how AI helps to reduce reading time and inter-reader variability. Results: AI has demonstrated sensitivity that is generally comparable to experienced radiologists, although specificity tends to be lower, potentially increasing false-positive rates. The clinical impact of these results requires validation in larger, prospective multicenter studies. AI is effective in identifying artifacts, assessing MRI quality, and assisting in diagnostic efficiency by providing second opinions and automating lesion detection. However, variability in study methodologies, datasets, and imaging protocols can impact AI's generalizability, limiting its broader clinical application. Conclusions: While AI shows significant promise in enhancing diagnostic accuracy and efficiency for csPCa detection, challenges remain, particularly with the generalizability of AI models. To improve AI robustness and integration into clinical practice, multicenter datasets and transparent reporting are essential. Further development, validation, and standardization are required for AI's widespread clinical adoption.
Keywords: artificial intelligence (AI); deep learning (DL); machine learning (ML); magnetic resonance imaging (MRI) of prostate; prostate cancer (PCa).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



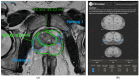
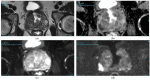
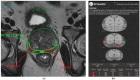
References
-
- Gillessen S., Armstrong A., Attard G., Beer T.M., Beltran H., Bjartell A., Bossi A., Briganti A., Bristow R.G., Bulbul M., et al. Management of Patients with Advanced Prostate Cancer: Report from the Advanced Prostate Cancer Consensus Conference 2021. Eur. Urol. 2022;82:115–141. doi: 10.1016/j.eururo.2022.04.002. - DOI - PubMed
-
- Saad F., Hotte S.J., Finelli A., Malone S., Niazi T., Noonan K., Shayegan B., So A.I., Danielson B., Basappa N.S., et al. Results from a Canadian consensus forum of key controversial areas in the management of advanced prostate cancer: Recommendations for Canadian healthcare providers. Can. Urol. Assoc. J. 2021;15:353–358. doi: 10.5489/cuaj.7347. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

