Comparative Analysis of RT-PCR and a Colloidal Gold Immunochromatographic Assay for SARS-CoV-2 Detection
- PMID: 40506934
- PMCID: PMC12154499
- DOI: 10.3390/diagnostics15111362
Comparative Analysis of RT-PCR and a Colloidal Gold Immunochromatographic Assay for SARS-CoV-2 Detection
Abstract
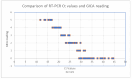
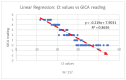
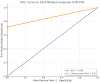
Background/Objectives: The COVID-19 pandemic has highlighted the urgent need for rapid, accurate, and accessible diagnostic testing to effectively manage and contain the spread of SARS-CoV-2. RT-PCR is widely recognized as the gold standard for SARS-CoV-2 detection due to its high sensitivity and specificity. However, RT-PCR testing requires specialized laboratory equipment, highly trained personnel, and extended processing times, which limits its feasibility for large-scale screening and point-of-care applications. This study aims to systematically evaluate the diagnostic performance of RT-PCR and a colloidal gold immunochromatographic assay (GICA). Methods: By comparing these two methods, we seek to determine a GICA's effectiveness as a complementary or alternative diagnostic tool, particularly in resource-limited settings and scenarios requiring rapid, large-scale testing. We assessed the following key clinical parameters: sensitivity, specificity, NPV, PPV, and accuracy. Additionally, we investigated the correlation between GICA signal intensity and RT-PCR Ct values using regression analysis, receiver operating characteristic curve analysis, and the calculated area under the curve. Results: Our findings indicate that while RT-PCR exhibits superior sensitivity, GICA results demonstrate a strong correlation with RT-PCR results and provide a rapid, cost-effective alternative for SARS-CoV-2 detection. Unlike RT-PCR, which requires extensive resources and prolonged turnaround times, a GICA delivers results within 20 min, making it a viable option for decentralized testing and real-time public health interventions. Conclusions: These results suggest that a GICA can serve as a complementary diagnostic tool alongside RT-PCR, particularly in resource-limited settings and high-throughput screening scenarios. By integrating GICAs into broader testing strategies, healthcare systems can enhance early detection efforts, improve accessibility to diagnostics, and strengthen pandemic response measures.
Keywords: COVID-19; SARS-CoV-2; colloidal gold immunochromatographic assay; cycle threshold; point of care; reverse transcription polymerase chain reaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
EDTA-K2 Improves the Detection Sensitivity of SARS-CoV-2 IgM and IgG Antibodies by Chelating Colloidal Gold in the Immunochromatographic Assay.Int J Nanomedicine. 2021 Jan 29;16:715-724. doi: 10.2147/IJN.S281594. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33542626 Free PMC article.
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
References
-
- Nichols J.D., Bogich T.L., Howerton E., Bjørnstad O.N., Borchering R.K., Ferrari M., Haran M., Jewell C., Pepin K.M., Probert W.J.M., et al. Strategic testing approaches for targeted disease monitoring can be used to inform pandemic decision-making. PLoS Biol. 2021;19:e3001307. doi: 10.1371/journal.pbio.3001307. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

