Regulatory Mechanisms and Therapeutic Targeting of PD-L1 Trafficking and Stability in Cancer Immunotherapy
- PMID: 40507229
- PMCID: PMC12153565
- DOI: 10.3390/cancers17111747
Regulatory Mechanisms and Therapeutic Targeting of PD-L1 Trafficking and Stability in Cancer Immunotherapy
Abstract
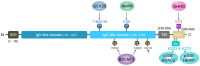
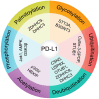
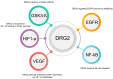
The PD-L1/PD-1 signaling axis is a pivotal regulator of T-cell activity and a key mechanism by which tumors evade immune surveillance. Inhibiting this pathway has resulted in significant anti-tumor responses, establishing immune checkpoint blockade (ICB) as a crucial component of modern cancer therapy. However, many patients with high PD-L1 expression do not respond to PD-1/PD-L1 blockade, underscoring the necessity for a deeper investigation into the mechanisms underlying this resistance. Recent studies have identified DRG2 as a critical modulator of anti-PD-1 therapeutic efficacy. While DRG2 depletion enhances IFN-γ signaling and increases the overall PD-L1 levels, it disrupts the recycling of endosomal PD-L1, resulting in reduced surface expression and impaired PD-1 interaction, ultimately compromising therapeutic outcomes. Furthermore, TRAPPC4, HIP1R, and CMTM6 help stabilize PD-L1 by preventing lysosome degradation. When depleted, these proteins have been shown to boost the body's immune response against tumors. Research into the complex regulatory mechanisms of PD-L1 suggests that targeting DRG2, TRAPPC4, HIP1R, and CMTM6 could enhance the effectiveness of PD-1/PD-L1 blockade therapies. This strategy could create exciting new possibilities for cancer immunotherapy and improve patient outcomes.
Keywords: CMTM6; HIP1R; PD-1 pathway; PD-L1 trafficking; Ras-associated binding proteins (Rab); TRAPPC4; immune checkpoint blockade; immune checkpoint blockade DRG2; post-translational modifications; tumor immune evasion.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
DRG2 is required for surface localization of PD-L1 and the efficacy of anti-PD-1 therapy.Cell Death Discov. 2024 May 27;10(1):260. doi: 10.1038/s41420-024-02027-x. Cell Death Discov. 2024. PMID: 38802348 Free PMC article.
-
DRG2 as a Biomarker to Enhance the Predictive Efficacy of PD-L1 Immunohistochemistry Assays.Biomedicines. 2024 Dec 29;13(1):56. doi: 10.3390/biomedicines13010056. Biomedicines. 2024. PMID: 39857640 Free PMC article.
-
Photo-induced crosslinked and anti-PD-L1 peptide incorporated liposomes to promote PD-L1 multivalent binding for effective immune checkpoint blockade therapy.Acta Pharm Sin B. 2024 Mar;14(3):1428-1440. doi: 10.1016/j.apsb.2023.09.007. Epub 2023 Sep 19. Acta Pharm Sin B. 2024. PMID: 38487005 Free PMC article.
-
Recent Advancements in the Mechanisms Underlying Resistance to PD-1/PD-L1 Blockade Immunotherapy.Cancers (Basel). 2021 Feb 7;13(4):663. doi: 10.3390/cancers13040663. Cancers (Basel). 2021. PMID: 33562324 Free PMC article. Review.
-
Programmed death receptor (PD-)1/PD-ligand (L)1 in urological cancers : the "all-around warrior" in immunotherapy.Mol Cancer. 2024 Sep 2;23(1):183. doi: 10.1186/s12943-024-02095-8. Mol Cancer. 2024. PMID: 39223527 Free PMC article. Review.
References
-
- Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

