JAK Inhibitors and Risk of Cancer in IBD Patients
- PMID: 40507276
- PMCID: PMC12153571
- DOI: 10.3390/cancers17111795
JAK Inhibitors and Risk of Cancer in IBD Patients
Abstract
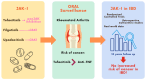
Janus kinase inhibitors, including tofacitinib, filgotinib, and upadacitinib, have emerged as effective therapeutic options for the management of inflammatory bowel diseases (IBDs). By targeting the JAK-STAT signaling pathway, these agents modulate immune responses and reduce inflammation. However, concerns regarding the potential risk of malignancy associated with their use have gained significant attention. The JAK-STAT pathway is not only critical for inflammatory signaling but also plays a pivotal role in cellular growth, differentiation, and tumor surveillance. Observational studies and clinical trial data in rheumatoid arthritis have reported malignancies, including non-melanoma skin cancer and solid tumors, in patients receiving JAK inhibitors, with evidence suggesting variable risks depending on the selectivity of the agent. Current evidence does not suggest an increased risk of oncogenesis in patients with IBDs. Balancing therapeutic efficacy with long-term safety requires ongoing vigilance; patient stratification based on risk factors; and tailored monitoring strategies to mitigate potential adverse effects, including malignancies, during JAK inhibitor therapy. Long-term follow-up data of up to 10 years offer reassuring evidence that JAK inhibitor therapy in IBD patients does not confer an increased risk of malignancies, supporting their continued use within appropriate clinical settings.
Keywords: Crohn’s disease; JAK inhibitor; cancer; filgotinib; inflammatory bowel disease; tofacitinib; ulcerative colitis; upadacitinib.
Conflict of interest statement
F.D. has served as a speaker for Abbvie, AnaptysBio, Ferring, Fresenius Kabi, Lilly, Takeda, Sandoz, Janssen, Galapagos, Tillots, and Omega Pharma; he also served as an advisory board member for Abbvie, Ferring, Fresenius Kabi, Galapagos, Janssen, Lilly, Takeda, and Nestlè. M.A. has received consulting fees from Nikkiso Europe, Mundipharma, Janssen, AbbVie, Ferring, and Pfizer. F.F. received consulting fees from Amgen, AbbVie, Lilly, Janssen and Pfizer. L.P.B. declares personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; stock options from CTMA. S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. F.B., I.F., A.Z., and T.L.P. declare no conflicts of interest.
Figures
Similar articles
-
Cancer Risk in IBD Patients Treated with JAK Inhibitors: Reassuring Evidence from Trials and Real-World Data.Cancers (Basel). 2025 Feb 21;17(5):735. doi: 10.3390/cancers17050735. Cancers (Basel). 2025. PMID: 40075582 Free PMC article. Review.
-
Comparative Efficacy and Safety of Three Janus Kinase Inhibitors in Ulcerative Colitis: A Real-World Multicentre Study in Japan.Aliment Pharmacol Ther. 2025 Feb;61(3):524-537. doi: 10.1111/apt.18406. Epub 2024 Nov 22. Aliment Pharmacol Ther. 2025. PMID: 39578704
-
Understanding the efficacy of individual Janus kinase inhibitors in the treatment of ulcerative colitis for future positioning in inflammatory bowel disease treatment.Immunol Med. 2023 Sep;46(3):121-130. doi: 10.1080/25785826.2023.2195522. Epub 2023 Apr 10. Immunol Med. 2023. PMID: 37036140 Review.
-
State-of-the-Art Evidence for Clinical Outcomes and Therapeutic Implications of Janus Kinase Inhibitors in Moderate-to-Severe Ulcerative Colitis: A Narrative Review.Pharmaceuticals (Basel). 2025 May 17;18(5):740. doi: 10.3390/ph18050740. Pharmaceuticals (Basel). 2025. PMID: 40430558 Free PMC article. Review.
-
Therapeutic inhibition of the JAK-STAT pathway in the treatment of inflammatory bowel disease.Cytokine Growth Factor Rev. 2024 Oct;79:1-15. doi: 10.1016/j.cytogfr.2024.07.008. Epub 2024 Aug 3. Cytokine Growth Factor Rev. 2024. PMID: 39179485 Review.
References
-
- Tofacitinib: Approved for Rheumatoid Arthritis. WHO Drug Inf. 2012;26:385.
-
- Commissioner Office of the FDA Approves New Treatment for Moderately to Severely Active Ulcerative Colitis. [(accessed on 26 January 2025)]. Available online: https://www.prnewswire.com/news-releases/fda-approves-new-treatment-for-....
Publication types
LinkOut - more resources
Full Text Sources

