Association of Pathologic Response and Adjuvant Chemotherapy with Survival in Resected Pancreatic Ductal Adenocarcinoma Following Neoadjuvant Therapy
- PMID: 40507279
- PMCID: PMC12153592
- DOI: 10.3390/cancers17111797
Association of Pathologic Response and Adjuvant Chemotherapy with Survival in Resected Pancreatic Ductal Adenocarcinoma Following Neoadjuvant Therapy
Abstract
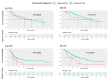
Background: In patients with curatively resected pancreatic adenocarcinoma who have undergone neoadjuvant chemotherapy (NACT), evidence supporting the benefit of additional adjuvant chemotherapy (ACT) remains limited. We aim to identify favorable factors contributing to survival benefits in resected pancreatic adenocarcinoma after NACT. Methods: This is a retrospective cohort study of pancreatic adenocarcinoma patients who underwent NACT followed by curative surgical resection between 2008 and 2023 at a single academic institution. Univariate and multivariable analyses were conducted to identify factors contributing to disease-free survival (DFS) and overall survival (OS). Results: A total of 230 patients with a median age of 68 years (IQR, 62-72 years) were included. All patients underwent curative surgical resection. Of these, 42% received neoadjuvant modified (m) FOLFIRINOX (96/230), 15% received gemcitabine plus nab-paclitaxel (GEM-NAB) (34/230), and 43% received gemcitabine, docetaxel, and capecitabine (GTX) (100/230). In univariate analysis, lower College of American Pathologists (CAP) tumor regression grade (TRG) (0-1 vs. 2-3, median DFS: 29.8 vs. 14.2 months, p = 0.0081) and receipt of ACT (Yes vs. No, median DFS: 22.2 vs. 12.4 months, p < 0.0001) demonstrated significant associations with superior DFS. Multivariable analysis identified receipt of ACT as an independent predictor of superior DFS (HR 0.55, 95% CI: 0.39-0.78, p = 0.0007) and OS (HR 0.49, 95% CI: 0.33-0.71, p = 0.0002). However, the NACT regimen (mFOLFIRINOX vs. GEM-NAB) and the transition between neoadjuvant and adjuvant therapy (de-escalation vs. continuation vs. change) did not correlate with DFS or OS. The duration of perioperative chemotherapy showed a trend toward improved survival outcomes, though not statistically significant (6 months vs. <6 months: DFS, 19.4 vs. 16.2 months, p = 0.1448; OS, 49.6 vs. 30.4 months, p = 0.0623). In the following subgroup analyses, receipt of ACT provided DFS/OS benefits in patients who did not achieve a major pathologic response, pN0, or R0 resection (DFS: p = 0.0003; OS: p < 0.0001). However, it did not provide DFS/OS benefits in those who achieved a major pathologic response with pN0/R0 to NACT (DFS: p = 0.8036; OS: p = 0.1877). Conclusions: In resected pancreatic adenocarcinoma following NACT, receiving ACT was associated with favorable survival outcomes. Additional ACT appears to benefit patients who did not achieve a major pathologic response (pN0 or R0) to neoadjuvant therapy, with limited benefit for those who achieved a major response with pN0/R0. The specific NACT regimen (mFOLFIRINOX vs. GEM-NAB) and changes in ACT from NACT did not significantly influence survival outcomes in our cohort.
Keywords: adjuvant chemotherapy; neoadjuvant therapy; pancreatic cancer; pathologic response.
Conflict of interest statement
J.Y., J.M.L, R.P, M.S., Y.K., J.C., I.I., N.L.N., M.M., J.W.D., J.B.F., S.E.H., J.M.F., A.J.S., P.J.H. and D.W.K. report no conflicts of interest related to this work. R.D.K. reports Consulting or Advisory Roles with Taiho Oncology, Exelixis, Servier, Pfizer, Roche/Genentech, AstraZeneca, AbbVie, Jazz Pharmaceuticals, and Eisai. Speakers’ Bureau: Incyte, AstraZeneca. J.M.P. reports Consulting or Advisory Roles with Ferranova, AstraZeneca, and Johnson & Johnson. T.B.d.C. reports consulting or advisory roles with AstraZeneca, Moderna Therapeutics, A2Bio. Speakers’ Bureau: AstraZeneca. Travel, Accommodations, Expenses: AstraZeneca, A2Bio, Ipsen. Research Funding: Astellas, Ipsen.
Figures


References
-
- SEER Cancer of the Pancreas—Cancer Stat Facts. [(accessed on 1 May 2025)]; Available online: https://seer.cancer.gov/statfacts/html/pancreas.html.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

