Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy
- PMID: 40507305
- PMCID: PMC12153556
- DOI: 10.3390/cancers17111824
Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy
Abstract
Background: Neuroblastoma (NB) is one of the most common solid tumors in children, still showing a high mortality rate despite recent advances in therapy. A recent breakthrough was the introduction of Dinutuximab beta, yielding further improvements in survival. Dinutuximab beta is an anti-GD2 monoclonal antibody that targets GD2 expressed on the cell surface of neuroblastoma cells. Evidence suggests that Dinutuximab beta combined with Nivolumab may offer an effective synergistic treatment approach.
Methods: In our center, immunotherapy was introduced in 2021 as part of maintenance treatment. The aim of this retrospective study was to analyze our data with a focus on the response, side effect profile and tolerability of Dinutuximab beta in HR and relapsed or refractory (r/r) NB.
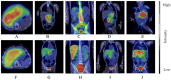
Results: Between 2021 and 2024, we treated 15 patients with neuroblastoma. Twelve patients had high-risk disease, of whom five received Dinutuximab beta as part of maintenance treatment according to protocol HR-NBL 1.8/SIOPEN. Two patients achieved complete remission after immunotherapy. One achieved long-lasting remission, while another relapsed. Three patients with inoperable tumors developed a partial response, but they relapsed and were diagnosed with metastases later. These patients subsequently initiated treatment with Temozolomide + Irinotecan in combination with Dinutuximab beta and also with Nivolumab as a relapse protocol. Therapeutic responses were assessed by the imaging, pathology and flow cytometry analysis of bone marrow. Apart from one complication (hypotension as part of capillary leak syndrome) subsiding spontaneously, no other severe adverse events were observed.
Conclusions: Our experiences confirm that immunotherapy, including Dinutuximab beta and Nivolumab, is safe and well tolerated. The standardization of the application of Dinutuximab beta and in combination with novel therapeutic agents in maintenance and refractory/relapsed cases may contribute to improved treatment outcome results.
Keywords: children; dinutuximab beta; neuroblastoma; nivolumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Campbell K., Siegel D.A., Umaretiya P.J., Dai S., Heczey A., Lupo P.J., Schraw J.M., Thompson T.D., Scheurer M.E., Foster J.H. A comprehensive analysis of neuroblastoma incidence, survival, and racial and ethnic disparities from 2001 to 2019. Pediatr. Blood Cancer. 2024;71:e30732. doi: 10.1002/pbc.30732. - DOI - PMC - PubMed
-
- Twist C.J., Schmidt M.L., Naranjo A., London W.B., Tenney S.C., Marachelian A., Shimada H., Collins M.H., Esiashvili N., Adkins E.S., et al. Maintaining outstanding outcomes using response- and biology-based therapy for intermediate-risk neuroblastoma: A report from the Children’s Oncology Group study ANBL0531. J. Clin. Oncol. 2019;37:3243–3255. doi: 10.1200/JCO.19.00919. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

