Clinical and Morphological Features of ER-Positive HER2-Negative Breast Tumors with PIK3CA Mutations in Russian Patients
- PMID: 40507317
- PMCID: PMC12153798
- DOI: 10.3390/cancers17111833
Clinical and Morphological Features of ER-Positive HER2-Negative Breast Tumors with PIK3CA Mutations in Russian Patients
Abstract
Background: Several targeted drugs have been recently approved for the treatment of PIK3CA-mutated hormone receptor-positive (HR+)/HER2-negative (HER2-) breast cancer (BC). This study aimed at a comprehensive evaluation of the spectrum of PIK3CA alterations in Russian BC patients.
Methods: The tumor material from 1872 patients with ER+/HER2- BC was tested by a combination of PCR-based methods.
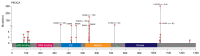
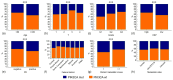
Results: Mutations were detected in 693/1872 (37%) cases, including 46 BC with two PIK3CA lesions. The three most common substitutions (E542K, E545K, and H1047R) were identified in 542/693 (78%) PIK3CA-mutated cases, while as many as 5.5-12% of identified mutations were not potentially detectable by common commercial kits. The study included patients of Slavic and non-Slavic ethnicities residing in regions with different climate conditions, however, these factors did not influence the distribution of PIK3CA mutations. The presence of PIK3CA variants was associated with older patient age at diagnosis (p = 0.0002), smaller tumor size (p = 0.005), lower grade (p = 0.005), Ki67 <20% (p = 0.0001) and progesterone receptor-positive status (p = 0.002) at the initial disease diagnosis, and fewer distant metastases at the time of the detection of BC spread (p = 0.0001). In a subgroup of 413 BC patients who received adjuvant tamoxifen or aromatase inhibitors, PIK3CA mutations were not associated with resistance to either type of treatment.
Conclusions: The results of this study highlight the need to extend the PIK3CA testing beyond the hotspot regions of this gene. Although PIK3CA alterations contribute to the pathogenesis of HR+/HER2- BC and represent a target for several novel drugs, they are not intrinsically associated with unfavorable clinical characteristics of this subtype of cancer disease.
Keywords: PIK3CA; hormone receptor-positive (HR+)/HER2-negative (HER2−) breast cancer; mutation.
Conflict of interest statement
Author Svetlana V. Odintsova was employed by the company EuroCityClinic LLC, The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- André F., Ciruelos E.M., Juric D., Loibl S., Campone M., Mayer I.A., Rubovszky G., Yamashita T., Kaufman B., Lu Y.S., et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021;32:208–217. doi: 10.1016/j.annonc.2020.11.011. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

