The Influence of Radioligand Therapy on Immunogenicity Against SARS-CoV-2-A Retrospective Single-Arm Cohort Study of Metastatic Prostate Cancer Patients Receiving PSMA Radioligand Therapy
- PMID: 40507346
- PMCID: PMC12153702
- DOI: 10.3390/cancers17111865
The Influence of Radioligand Therapy on Immunogenicity Against SARS-CoV-2-A Retrospective Single-Arm Cohort Study of Metastatic Prostate Cancer Patients Receiving PSMA Radioligand Therapy
Abstract
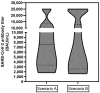
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a rising threat for immunocompromised cancer patients. The reduced immune defense may be a result of the malignancy itself or a side effect of therapy. While many chemotherapies can severely diminish the effect of vaccines against SARS-CoV-2, the effect of radioligand therapy has not yet been studied so far. Methods: In our database, 64 patient records of patients with metastatic castration-resistant prostate cancer that were treated with PSMA-directed radioligand therapy (PRLT) were randomly selected and checked for specific information (vaccination status, past corona virus disease 2019 (COVID-19) infections, the period between PRLT and vaccination, and antibody titers). A total of 30 patient records had sufficient information to examine the interference between PRLT and the vaccination against SARS-CoV-2. Results: In the analyzed cohort, 96.7% of the patients achieved seroconversion after receiving-on average-the third (booster) vaccination against SARS-CoV-2 and two PRLT cycles with average administered activities of 16.1 ± 7.2 GBq (435.1 ± 194.6 mCi) of lutetium-177 and 13.7 ± 6.6 MBq (0.37 ± 0.18 mCi) of actinium-225 (as part of 'TANDEM therapies') per patient. Conclusions: In the reviewed population, neither the initial response nor the maintenance of a positive immune response against the SARS-CoV-2 virus was undesirably affected by PRLT. The seroconversion rate and the absolute immune titers (in many cases >25,000 BAU/mL) are comparable to the normal population. This result implies the clinically important conclusion that neither an initial nor a booster vaccination against COVID-19 must be postponed if a PRLT is planned (and vice versa).
Keywords: COVID-19; PSMA; SARS-CoV-2; clinical care; humoral response; immunization; prostate cancer; radioligand therapy; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer.Eur Urol. 2021 Jul;80(1):82-94. doi: 10.1016/j.eururo.2021.03.004. Epub 2021 Apr 8. Eur Urol. 2021. PMID: 33840558 Free PMC article.
-
Combined 177 Lu-PSMA-617 PRLT and abiraterone acetate versus 177 Lu-PSMA-617 PRLT monotherapy in metastatic castration-resistant prostate cancer: An observational study comparing the response and durability.Prostate. 2021 Nov;81(15):1225-1234. doi: 10.1002/pros.24219. Epub 2021 Sep 1. Prostate. 2021. PMID: 34469602
-
Prostate-Specific Membrane Antigen Radioligand Therapy Using 177Lu-PSMA I&T and 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparison of Safety, Biodistribution, and Dosimetry.J Nucl Med. 2022 Aug;63(8):1199-1207. doi: 10.2967/jnumed.121.262713. Epub 2021 Dec 9. J Nucl Med. 2022. PMID: 34887335 Free PMC article.
-
Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort.Br J Radiol. 2019 Dec;92(1104):20190380. doi: 10.1259/bjr.20190380. Epub 2019 Nov 1. Br J Radiol. 2019. PMID: 31600089 Free PMC article.
-
PSMA-targeted radioligand therapy in prostate cancer: current status and future prospects.Expert Rev Anticancer Ther. 2023 Jul-Dec;23(9):959-975. doi: 10.1080/14737140.2023.2247562. Epub 2023 Aug 28. Expert Rev Anticancer Ther. 2023. PMID: 37565281 Review.
References
-
- Garassino M.C., Vyas M., de Vries E.G.E., Kanesvaran R., Giuliani R., Peters S., European Society for Medical Oncology The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann. Oncol. 2021;32:579–581. doi: 10.1016/j.annonc.2021.01.068. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

