Randomized, Cross over, Multicenter, Single-Blind Study Comparing Citicoline 500 mg/Homotaurine 50 mg/Vitamin B3 54 mg/Pyrroloquinoline Quinone 5 mg (Neuprozin Mito®) and Citicoline 800 mg (Cebrolux®) on Pattern Electroretinogram (PERG) and Quality of Life in Patients with Primary Open-Angle Glaucoma with Well-Controlled Intraocular Pressure
- PMID: 40507536
- PMCID: PMC12155607
- DOI: 10.3390/jcm14113774
Randomized, Cross over, Multicenter, Single-Blind Study Comparing Citicoline 500 mg/Homotaurine 50 mg/Vitamin B3 54 mg/Pyrroloquinoline Quinone 5 mg (Neuprozin Mito®) and Citicoline 800 mg (Cebrolux®) on Pattern Electroretinogram (PERG) and Quality of Life in Patients with Primary Open-Angle Glaucoma with Well-Controlled Intraocular Pressure
Abstract
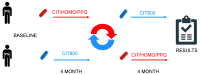
Background/Objectives: To evaluate the neuromodulative effects of oral intake of a fixed combination of citicoline 500 mg plus homotaurine 50 mg plus vitamin B3 54 mg plus pyrroloquinoline quinone (CIT/HOMO/B3/PPQ) or of citicoline 800 mg alone (CIT800) on retinal ganglion cell (RGC) function in glaucoma patients by pattern electroretinogram (PERG) and to investigate the effects on quality of life and visual function. Methods: Consecutive patients with primary open-angle glaucoma with controlled IOP (<18 mmHg) receiving prostaglandin analogues as monotherapy; with two reliable visual fields (Humphrey 24-2 SITA Standard) per year in the last 2 years; and an early to moderate visual field defect (MD < -12 dB) were randomized to: arm A. topical therapy + CIT/HOMO/B3/PPQ for 4 months, followed by 4 months of topical therapy + CIT800; and arm B. topical therapy + CIT800 for 4 months, then topical therapy + CIT/HOMO/B3/PPG for 4 months. Patients were examined at month 0, 4, and 8. Complete ocular examination, visual field test, PERG, and quality of life assessment (NEI-VFQ25) were performed at each visit. Results: Forty patients were selected and completed the study, and none developed or reported an adverse event. The overall mean age was 64.2 (±7.7) years, 27 were male. At the end of the intake period of both products, patients exhibited higher P50 and N95-wave amplitudes and shorter latencies compared to baseline. The crossover analysis found that PERG parameters were better when patients received the CIT/HOMO/B3/PQQ combination with a statistically significant shorter peak time of 1.24 ms (95% CI, 0.37 to 2.10; p = 0.006) in the central P50 wave, 1.32 ms (95% CI, 0.44 to 2.22; p = 0.004) in the inferior P50 wave, and 1.70 ms (95% CI, 0.09 to 3.31; p = 0.038) in the inferior N95 wave; and a statistically significant increase of 0.35 µV (95% CI, 0.10 to 0.60; p = 0.006) in the superior N95 amplitude. The crossover analysis did not reveal any significant differences between the intake of CIT800 and CIT/HOMO/B3/PQQ in terms of visual acuity or IOP. During the intake of CIT/HOMO/B3/PQQ, a significant improvement was observed in the total mean score (p = 0.004), in the general health scale (GH, p = 0.01), in the color vision scale (p = 0.006), and in the peripheral vision scale (p = 0.001). Conclusions: The present study has shown that the addition of CIT/HOMO/B3/PQQ in early glaucoma improves PERG parameters and quality of life, likely by slowing down RGC aging and enhancing mitochondrial function more significantly than citicoline 800 mg alone.
Keywords: citicoline; glaucoma; homotaurine; neuroprotection; pattern electroretinogram; pyrroloquinoline quinone; quality of life; visual field.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kass M.A., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., II, Wilson M.R., Gordon M.O., the Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous