Profiling the Tox21 Compound Library for Their Inhibitory Effects on Cytochrome P450 Enzymes
- PMID: 40507787
- PMCID: PMC12155096
- DOI: 10.3390/ijms26114976
Profiling the Tox21 Compound Library for Their Inhibitory Effects on Cytochrome P450 Enzymes
Abstract
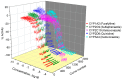
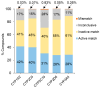
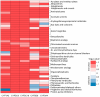
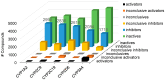
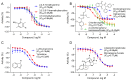
Cytochrome P450 (CYP) enzymes are membrane-bound hemoproteins crucial for drug and xenobiotic metabolism. While more than 50 CYPs have been identified in humans, the isoforms from CYP1, 2, and 3 families contribute to the metabolism of about 80% of clinically approved drugs. To evaluate the effects of environmental chemicals on the activities of these important CYP enzyme families, we screened the Tox21 10K compound library to identify chemicals that inhibit CYP1A2, 2C9, 2C19, 2D6, and 3A4 enzymes. The data obtained from these five screenings were analyzed to reveal the structural classes responsible for inhibiting multiple and/or selective CYPs. Some known structural compound classes exhibiting pan-CYP inhibition, such as azole fungicides, along with established clinical inhibitors of CYPs, including erythromycin and verapamil inhibiting CYP3A4 and paroxetine and terbinafine inhibiting CYP2D6, were all confirmed in the current study. In addition, some selective CYP inhibitors, previously unknown but with potent activity (IC50 values < 1 µM), were identified. Examples included yohimbine, an indole alkaloid, and loteprednol, a corticosteroid, which showed inhibitory activity in CYP2D6 and 3A4 assays, respectively. These findings suggest that assessment of a candidate compound's impact on CYP function may allow pre-emptive mitigation of potential adverse reactions and toxicity during drug development or toxicological characterization of environmental chemicals.
Keywords: CYP inhibitors; CYP1A2; CYP2C19; CYP2C9; CYP2D6; CYP3A4; cytochrome P450 (CYP); quantitative high-throughput screening (qHTS).
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Goedtke L., Sprenger H., Hofmann U., Schmidt F.F., Hammer H.S., Zanger U.M., Poetz O., Seidel A., Braeuning A., Hessel-Pras S. Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells. Int. J. Mol. Sci. 2020;22:372. doi: 10.3390/ijms22010372. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

