Repeated Valproic Acid Administration Fundamentally Ameliorated Cisplatin-Induced Mechanical Allodynia in Rats
- PMID: 40507788
- PMCID: PMC12155109
- DOI: 10.3390/ijms26114977
Repeated Valproic Acid Administration Fundamentally Ameliorated Cisplatin-Induced Mechanical Allodynia in Rats
Abstract
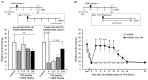
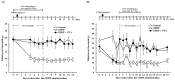
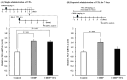
Cisplatin (cis-diamminedichloro-platinum; CDDP) is a chemotherapeutic agent that frequently induces peripheral neuropathy characterized by mechanical allodynia. Herein, we aimed to determine the effects of valproic acid (VPA) on cisplatin-induced mechanical allodynia in rats and elucidate the underlying mechanisms. A single administration of VPA (150 mg/kg) transiently suppressed CDDP-induced mechanical allodynia, correlating with serum VPA concentrations. Repeated VPA administration before or after the onset of CDDP-induced mechanical allodynia significantly attenuated allodynia even after VPA discontinuation, suggesting fundamental treatment potential. Mechanistically, CDDP increased the expression of neurokinin 1 receptor (NK1R) mRNA in the dorsal horn of the spinal cord, and this increased expression was suppressed by repeated VPA administration. Treatment with an NK1R antagonist alleviated CDDP-induced mechanical allodynia, indicating the involvement of NK1R in allodynia. In vitro assays revealed that VPA did not affect the cytotoxicity of CDDP in Walker 256 cells, suggesting that VPA does not interfere with the antitumor activity of CDDP. Overall, repeated VPA administration may fundamentally ameliorate CDDP-induced peripheral neuropathy by suppressing the CDDP-induced increased NK1R expression without compromising the antitumor effects of CDDP. These findings provide insights into the potential use of VPA as a therapeutic agent for managing CDDP-induced peripheral neuropathy.
Keywords: cisplatin; mechanical allodynia; valproic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kenmotsu H., Yamamoto N., Misumi T., Yoh K., Saito H., Sugawara S., Yamazaki K., Nakagawa K., Sugio K., Seto T., et al. Five-Year Overall Survival Analysis of the JIPANG Study: Pemetrexed or Vinorelbine Plus Cisplatin for Resected Stage II-IIIA Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023;41:5242–5246. doi: 10.1200/JCO.23.00179. - DOI - PubMed
-
- Baselga J., Gómez P., Greil R., Braga S., Climent M.A., Wardley A.M., Kaufman B., Stemmer S.M., Pego A., Chan A., et al. Randomized Phase II Study of the Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Cetuximab with Cisplatin versus Cisplatin Alone in Patients with Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

