RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments
- PMID: 40507804
- PMCID: PMC12155363
- DOI: 10.3390/ijms26114990
RANKL Drives Bone Metastasis in Mammary Cancer: Protective Effects of Anti-Resorptive Treatments
Abstract
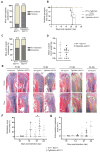
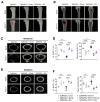
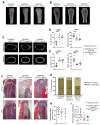
Receptor activator of nuclear factor-κB ligand (RANKL) is essential for osteoclast formation and bone resorption, in osteolytic conditions such as osteoporosis and bone metastases. However, its role in metastasis progression remains incompletely understood. Herein, we examined whether the overexpression of human RANKL in transgenic mice (TgRANKL) affects their susceptibility to breast cancer bone metastasis compared to their wild-type (WT) littermates. Bone metastasis was induced by injecting EO771 mouse mammary adenocarcinoma cells into the caudal artery of syngeneic WT and TgRANKL mice. RANKL overexpression led to an earlier onset and increased burden of bone metastasis in EO771-bearing TgRANKL mice compared to WT mice. It also exacerbated the bone destruction caused by metastasis-associated osteolysis. The prophylactic inhibition of RANKL activity with denosumab, a monoclonal antibody targeting human RANKL, prevented osteolysis and significantly reduced the incidence and progression of bone metastases in TgRANKL mice. However, the therapeutic denosumab treatment had no effect on metastasis incidence or tumor burden, although it alleviated osteolysis. The treatment with zoledronic acid, an anti-resorptive agent inhibiting osteoclast activity, yielded results similar to those of denosumab. These findings emphasize the significance of initiating early treatment with anti-resorptive agents such as denosumab or zoledronic acid to reduce the risk of bone metastasis in patients at high risk.
Keywords: RANKL; bone metastasis; breast cancer; mouse models; pre-clinical models.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Huang J.-F., Shen J., Li X., Rengan R., Silvestris N., Wang M., Derosa L., Zheng X., Belli A., Zhang X.-L., et al. Incidence of Patients with Bone Metastases at Diagnosis of Solid Tumors in Adults: A Large Population-Based Study. Ann. Transl. Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. - DOI - PMC - PubMed
-
- Coleman R.E., Brown J., Holen I. Abeloff’s Clinical Oncology. Elsevier; Amsterdam, The Netherlands: 2020. Bone Metastases; pp. 809–830.e3.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

