Opioid-Induced Regulation of Cortical Circular- Grin2b _011731 Is Associated with Regulation of circGrin2b Sponge Target miR-26b-3p
- PMID: 40507822
- PMCID: PMC12154416
- DOI: 10.3390/ijms26115010
Opioid-Induced Regulation of Cortical Circular- Grin2b _011731 Is Associated with Regulation of circGrin2b Sponge Target miR-26b-3p
Abstract
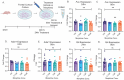
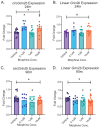
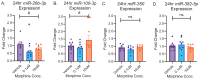
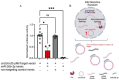
Opioid use induces neurobiological adaptations throughout mesolimbic brain regions, such as the orbitofrontal cortex (OFC), which mediates decision-making and emotional-cognitive regulation. Previously, we showed that a circular RNA (circRNA) species, rno_circGrin2b_011731 (circGrin2b), is upregulated in the OFC of rats following chronic self-administration (SA) of the opioid heroin. circGrin2b is derived from Grin2b, which encodes the regulatory subunit of the glutamate ionotropic NMDA receptor, GluN2B. However, the upstream regulatory mechanisms of circGrin2b biogenesis and the downstream consequences of circGrin2b dysregulation remain unknown. We hypothesized that opioid-induced elevation of circGrin2b is accompanied by regulation of circRNA biogenesis enzymes, and that circGrin2b may sponge microRNAs (miRNAs), as miRNA sponging is a well-described characteristic of circRNAs. To test these hypotheses, we established an in vitro primary cortical cell culture model to examine alterations in circGrin2b expression following exposure to the opioid morphine. We measured mRNA expression of known circRNA splicing factors and observed significant downregulation of Fused in Sarcoma (Fus), a negative regulator of circRNA biogenesis, following 90 min or 24 h of morphine exposure. Downregulation of Fus at 24 h post-morphine was accompanied by upregulation of circGrin2b and downregulation of miR-26b-3p, a predicted miRNA target of circGrin2b. Luciferase reporter assays confirmed interaction of miR-26b-3p with circGrin2b. Finally, we report a significant negative relationship between circGrin2b and miR-26b-3p expression in the OFC of rats following heroin SA. We conclude that regulation of circGrin2b is an opioid-induced neuroadaptation that may impact downstream signaling of miRNA pathways in the frontal cortex.
Keywords: circular RNA; microRNA; morphine; opioids; splicing.
Conflict of interest statement
The authors declare that we do not have any conflicts of interest.
Figures

References
-
- Ahmad F.B., Cisewski J.A., Rossen L.M., Sutton P. National Center for Health Statistics. [(accessed on 4 September 2024)];2022 Available online: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
-
- Centers for Disease Control and Prevention Multiple Cause of Death Files, National Center for Health Statistics. 1999–2019. [(accessed on 27 September 2021)]; Available online: https://wonder.cdc.gov/
-
- Hedegaard H., Miniño A.M., Warner M. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Briefs. 2021;406:1–8. - PubMed
-
- Smyth B.P., Barry J., Keenan E., Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir. Med. J. 2010;103:176–179. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

