Successful Management of C3 Glomerulopathy Recurrence Post-Kidney Transplantation with Iptacopan: A Case Report
- PMID: 40507864
- PMCID: PMC12154424
- DOI: 10.3390/ijms26115053
Successful Management of C3 Glomerulopathy Recurrence Post-Kidney Transplantation with Iptacopan: A Case Report
Abstract
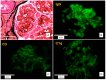
C3 glomerulopathy (C3G) is the predominant cause of complement-mediated membranoproliferative glomerulonephritis and is considered a rare disorder caused by genetic or acquired dysregulation of the alternative complement pathway. There are no established treatment guidelines for treating kidney-transplanted recipients with C3G recurrence, as they are already on immunosuppressive protocols. Furthermore, non-complement-specific immunosuppressive drugs appear to offer limited benefits for patients with C3G in native kidneys. Therefore, modulating the complement system appears to be the most effective strategy for this specific patient population. We describe the use of Iptacopan in a 38-year-old kidney-transplanted patient with C3G recurrence. Iptacopan was associated with a significant and striking improvement in the patient's clinical and laboratories status. A follow-up kidney biopsy performed 5 months after the initiation of Iptacopan revealed a reduction in endocapillary, extracapillary and mesangial hypercellularity, along with a decreased extent of parietal proteinaceous deposits observed on light microscopy. The direct control of the complement dysregulation underlying the pathogenesis of C3G with Iptacopan was accompanied by improvements in clinical, laboratory and histological features, with demonstrated reduced disease activity and slowed disease progression. Therefore, the case report described is intended to shed light on the potential role of new AP complement blockers in the treatment of C3G.
Keywords: C3 glomerulopathy; Iptacopan; complement system; innate immunity.
Conflict of interest statement
The authors declare no conflicts of interest. Novartis has not influenced the content of the publication.
Figures




References
-
- Caravaca-Fontán F., Díaz-Encarnación M.M., Lucientes L., Cavero T., Cabello V., Ariceta G., Quintana L.F., Marco H., Barros X., Ramos N., et al. Mycophenolate Mofetil in C3 Glomerulopathy and Pathogenic Drivers of the Disease. Clin. J. Am. Soc. Nephrol. 2020;15:1287–1298. doi: 10.2215/CJN.15241219. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

