Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects
- PMID: 40507968
- PMCID: PMC12155405
- DOI: 10.3390/ijms26115160
Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects
Abstract
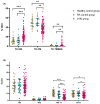
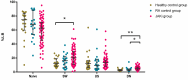
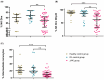
Recent concerns regarding the safety of Janus kinase inhibitors (JAKis) have prompted investigation into their impact on immune cell subsets in rheumatoid arthritis (RA) patients. This study aims to analyse alterations in immune cell populations induced by JAKis that may contribute to adverse events, such as infections or malignancies. This study included 78 RA patients meeting ACR/EULAR criteria with an established treatment with JAKis (tofacitinib, baricitinib, upadacitinib, or filgotinib), 20 healthy donors, and 20 RA patients treated with biological disease-modifying antirheumatic drugs (bDMARDs). Peripheral blood mononuclear cells (PBMCs) were immunophenotyped directly after isolation using multiparametric flow cytometry to characterise innate and adaptive immune-cell subsets. JAKi-treated patients showed a significant reduction in cytotoxic NK Dim (CD3-CD56+CD16+) cells and in the percentage of NK Dim cells expressing the activation marker Nkp30. In CD4+ T cells, the percentage of Th17 (CD3+CD4+CD45RA+CCR6+CXCR3-), Th1-17 (CD3+CD4+CD45RA+CCR6+CXCR3+), and central memory (CM, CD3+CD4+CD45RA+CD62L+) cells was lower in the JAKi group, while effector memory (EM, CD3+CD4+CD45RA-CD62L-) and terminally differentiated CD45RA (TEMRA, CD3+CD4+CD45RA+CD62L-) T helper cells were increased compared to healthy and bDMARD-treated controls. The reduction in NK Dim and Th1-17 cells and the increase in exhausted Th subsets suggest a potential compromise in antiviral immunity and balanced immune responses in JAKi-treated RA patients. These alterations may contribute to an increased risk of infections or malignancies.
Keywords: JAKi; NK cells; Th cells; flow cytometry; monocytes; rheumatoid arthritis.
Conflict of interest statement
Dr. Ricardo Blanco received grants/research support from AbbVie, MSD, and Roche and received funds for consultation/participation fees in a company-sponsored speaker’s bureau from AbbVie, Pfizer, Roche, Bristol-Myers, Lilly, Galapagos, Novartis, Janssen, GSK, and MSD. Dr. José Luis Martín-Varillas received grants/research support from AbbVie, Pfizer, Lilly, Celgene, Janssen, and UCB Pharma. The rest of the authors declare no conflicts of interest regarding this work.
Figures
References
-
- Yu C., Jin S., Wang Y., Jiang N., Wu C., Wang Q., Tian X., Li M., Zeng X. Remission Rate and Predictors of Remission in Patients with Rheumatoid Arthritis under Treat-to-Target Strategy in Real-World Studies: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2019;38:727–738. doi: 10.1007/s10067-018-4340-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

