Regulation and Function of Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): The Role of the SRIF System in Macrophage Regulation
- PMID: 40508145
- PMCID: PMC12155148
- DOI: 10.3390/ijms26115336
Regulation and Function of Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): The Role of the SRIF System in Macrophage Regulation
Abstract
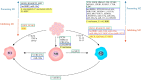
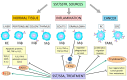
Colorectal cancer (CRC) remains the leading cause of morbidity and mortality for both men and women worldwide. Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment (TME) of solid tumors, including CRC. These macrophages are found in the pro-inflammatory M1 and anti-inflammatory M2 forms, with the latter increasingly recognized for its tumor-promoting phenotypes. Many signaling molecules and pathways, including AMPK, EGFR, STAT3/6, mTOR, NF-κB, MAPK/ERK, and HIFs, are involved in regulating TAM polarization. Consequently, researchers are investigating several potential predictive and prognostic markers, and novel TAM-based therapeutic targets, especially in combination therapies for CRC. Macrophages of the gastrointestinal tract, including the normal colon and rectum, produce growth hormone-releasing inhibitory peptide/somatostatin (SRIF/SST) and five SST receptors (SSTRs, SST1-5). While the immunosuppressive function of the SRIF system is primarily known for various tissues, its role within CRC-associated TAMs remains underexplored. This review focuses on the following three aspects of TAMs: first, the role of macrophages in the normal colon and rectum within the broader context of macrophage biology; second, the various bioactive factors and signaling pathways associated with TAM function, along with potential strategies targeting TAMs in CRC; and third, the interaction between the SRIF system and macrophages in both normal tissues and the CRC microenvironment.
Keywords: colorectal cancer; macrophages; polarization; role of SRIF system in immune system; somatostatin; somatostatin receptors; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Gab2 promotes the growth of colorectal cancer by regulating the M2 polarization of tumor‑associated macrophages.Int J Mol Med. 2024 Jan;53(1):3. doi: 10.3892/ijmm.2023.5327. Epub 2023 Nov 8. Int J Mol Med. 2024. PMID: 37937666 Free PMC article.
-
CD133-containing microvesicles promote cancer progression by inducing M2-like tumor-associated macrophage polarization in the tumor microenvironment of colorectal cancer.Carcinogenesis. 2024 May 19;45(5):300-310. doi: 10.1093/carcin/bgad093. Carcinogenesis. 2024. PMID: 38085813
-
CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling.J Exp Clin Cancer Res. 2020 Jul 11;39(1):132. doi: 10.1186/s13046-020-01637-4. J Exp Clin Cancer Res. 2020. PMID: 32653013 Free PMC article.
-
Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis.Int J Mol Sci. 2021 Aug 6;22(16):8470. doi: 10.3390/ijms22168470. Int J Mol Sci. 2021. PMID: 34445193 Free PMC article. Review.
-
Natural compounds modulate the mechanism of action of tumour-associated macrophages against colorectal cancer: a review.J Cancer Res Clin Oncol. 2024 Nov 15;150(11):502. doi: 10.1007/s00432-024-06022-8. J Cancer Res Clin Oncol. 2024. PMID: 39546016 Free PMC article. Review.
References
-
- Patel S.G., Karlitz J.J., Yen T., Lieu C.H., Boland C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022;7:262–274. doi: 10.1016/S2468-1253(21)00426-X. - DOI - PubMed
-
- Sung H., Siegel R.L., Laversanne M., Jiang C., Morgan E., Zahwe M., Cao Y., Bray F., Jemal A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025;26:51–63. doi: 10.1016/S1470-2045(24)00600-4. - DOI - PMC - PubMed
-
- Ugai T., Väyrynen J.P., Lau M.C., Borowsky J., Akimoto N., Väyrynen S.A., Zhao M., Zhong R., Haruki K., Costa A.D., et al. Immune cell profiles in the tumor microenvironment of early-onset, intermediate-onset, and later-onset colorectal cancer. Cancer Immunol. Immunother. 2022;71:933–942. doi: 10.1007/s00262-021-03056-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

