Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo
- PMID: 40508166
- PMCID: PMC12154752
- DOI: 10.3390/ijms26115357
Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo
Abstract
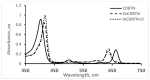
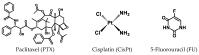
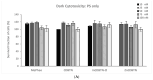
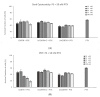
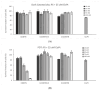
In this article, the synthesis and characterization of chlorin-based photosensitizers for potential applications in photodynamic therapy (PDT) of triple-negative breast cancer (TNBC) are described. The photodynamic efficacy of the synthesized chlorin-desthiobiotin (CDBTN) conjugate and its zinc and indium complexes were compared with the starting unconjugated precursor methyl pheophorbide, and assessed in a TNBC cell line in vitro. The chlorin-desthiobiotin complex aims to target the vitamin receptors upregulated in malignant cancer cells. The synthesized CDBTN was combined with chemotherapeutic agents (paclitaxel, cisplatin or fluorouracil) to evaluate their binary photodynamic efficacy. Cell survival assay in vitro indicated that the chlorin-vitamin conjugate CDBTN-alone and in combination with paclitaxel or fluorouracil-is photoactive against the TNBC cell line, but not when combined with cisplatin. The combination index (CI) calculated using the Chou-Talalay method indicated synergism of CDBTN and fluorouracil combination, aligning with the in vitro assay. The photodynamic cytotoxicity of CDBTN was also evaluated in vivo using the hydra as a novel model organism. This study is the first to show the use of the aquatic hydra organism in assessing photodynamic activity of the photosensitizer alone or in combination with chemotherapeutic agents. In vivo results with hydras indicated that the CDBTN-cisplatin combination is more phototoxic than CDBTN-paclitaxel or CDBTN-fluorouracil binary treatment. With the proper adjustment of concentration and light dosage, the synthesized photosensitizer can provide promising application in binary chemotherapy PDT treatment of TNBC.
Keywords: PDT; TNBC; chemophotodynamic therapy; chemotherapy; chlorin-vitamin conjugates; cisplatin; fluorouracil; hydra model organism; paclitaxel; photodynamic therapy; photosensitizers; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
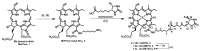






References
-
- Allamyradov Y., ben Yosef J., Annamuradov B., Ateyeh M., Street C., Whipple H., Er A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem. 2024;4:434–461. doi: 10.3390/photochem4040027. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

