Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection
- PMID: 40508173
- PMCID: PMC12154513
- DOI: 10.3390/ijms26115364
Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection
Abstract
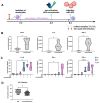
A diverse bacterial community colonizes the respiratory system, including commensals such as Staphylococcus epidermidis (S. epidermidis) and Streptococcus salivarius (S. salivarius), as well as facultative pathogens like Staphylococcus aureus (S. aureus). This study aimed to establish a colonized cell culture model to investigate the impact of these bacteria on influenza A virus (IAV) infection. Respiratory epithelial cells were exposed to S. epidermidis, S. salivarius, or S. aureus, using either live or heat-inactivated bacteria, followed by IAV infection. Cell integrity was assessed microscopically, cytotoxicity was measured via LDH assay, and inflammatory responses were analyzed through cytokine expression. Additionally, macrophage function was examined in response to bacterial colonization and IAV infection. While commensals maintained epithelial integrity for 48 h, S. aureus induced severe cell damage and death. The most pronounced epithelial destruction was caused by coinfection with S. aureus and IAV. Notably, commensals did not confer protection against IAV but instead enhanced epithelial inflammation. These effects were dependent on live bacteria, as inactivated bacteria had no impact. However, prior exposure to S. epidermidis and S. salivarius improved macrophage-mediated immune responses against IAV. These findings suggest that while individual commensals do not directly protect epithelial cells, they may contribute to immune training and enhance lung defense mechanisms.
Keywords: cell culture model; commensal bacteria; cytotoxicity; facultative pathogenic bacteria; influenza A virus; macrophages; respiratory microbiome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Oberbach A., Schlichting N., Hagl C., Lehmann S., Kullnick Y., Friedrich M., Köhl U., Horn F., Kumbhari V., Löffler B., et al. Four decades of experience of prosthetic valve endocarditis reflect a high variety of diverse pathogens. Cardiovasc. Res. 2023;119:410–428. doi: 10.1093/cvr/cvac055. - DOI - PubMed
-
- Liu Q., Liu Q., Meng H., Lv H., Liu Y., Liu J., Wang H., He L., Qin J., Wang Y., et al. Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production. Cell Host Microbe. 2020;27:68–78.e5. doi: 10.1016/j.chom.2019.11.003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

