Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids
- PMID: 40508178
- PMCID: PMC12154467
- DOI: 10.3390/ijms26115368
Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids
Abstract
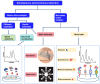
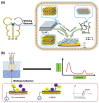
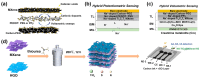
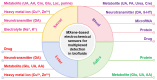
Detection of multiple analytes in biofluids is of significance for early disease diagnosis, effective treatment monitoring, and accurate prognostic assessment. Electrochemical sensors have emerged as a promising tool for the multiplexed detection of biofluids due to their low cost, high sensitivity, and rapid response. Two-dimensional transition metal carbon/nitride MXene, which has the advantages of a large specific surface area, good electrical conductivity, and abundant surface functional groups, has received increasing attention in the electrochemical sensing field. This paper systematically reviews the advances of MXene-based electrochemical sensors for multiplexed detection in biofluids, emphasizing the design of MXene-based electrode materials as well as the strategies for distinguishing multiple signals during simultaneous electrochemical analysis. In addition, this paper critically analyzes the existing challenges of MXene-based electrochemical sensors for multiplexed detection of biofluids and proposes future development directions for this field. The ultimate goal is to improve biofluid multiplexed detection technology for clinical medical applications.
Keywords: MXene; biofluids; electrochemical sensors; multiplexed; simultaneous.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

