Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior
- PMID: 40508517
- PMCID: PMC12156935
- DOI: 10.3390/ma18112520
Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior
Abstract
Photochromic materials have attracted widespread attention due to their potential applications in optical information storage, optoelectronic devices, and fluorescence probes. As a typical photochromic system, diarylethene derivatives are considered one of the most promising photochromic materials due to their outstanding photostability and significant bistable properties. Based on an aggregation-induced emission (AIE) mechanism, this study employed a molecular structural engineering strategy to design and synthesize a series of diarylethene derivatives containing ethyl benzoate substituents. A systematic investigation of the structure-activity relationship between their photochromic behavior and AIE characteristics revealed a dual-state light response mechanism in the solid and solution states. This study demonstrates that the target compounds exhibited significant photochromic responses under UV-visible light irradiation, with enhanced emission in the solid state compared to the solution state, confirming the remarkable enhancement effect of AIE on aggregation. Structural characterization techniques such as nuclear magnetic resonance spectroscopy (NMR) and high-resolution mass spectrometry (H RMS) were employed to elucidate the correlation between molecular conformation and photophysical properties. Furthermore, these materials demonstrated potential for multi-level anti-counterfeiting, high-density optical storage, and bioimaging applications, providing experimental foundations for the development of novel multifunctional photochromic materials.
Keywords: aggregation-induced emission effect; diarylethene derivatives; photophysical properties; photoresponsivity properties.
Conflict of interest statement
Author Haoyuan Yu was employed by the company Shanghai StarryInk Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
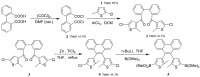

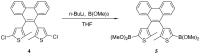
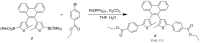

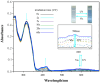


References
-
- Luo M., Zhao J., Liu Y., Mao Z., Wang S., Chi Z. All-visible-light triggered photoswitch of dithienylethene derivatives with molecular conformation changes exceeding 5 Å. Adv. Funct. Mater. 2022;33:2211009. doi: 10.1002/adfm.202211009. - DOI
-
- Li Z., Gao X., Zhang H., Ma X., Liu Y., Guo H., Yin J. Efficient blue light-responsive dithienylethenes with exceptional photochromic performance. Chin. Chem. Lett. 2023;34:107645. doi: 10.1016/j.cclet.2022.06.068. - DOI
-
- Li R.-J., Han M., Tessarolo J., Holstein J.J., Lübben J., Dittrich B., Volkmann C., Finze M., Jenne C., Clever G.H. Successive photoswitching and derivatization effects in photochromic dithienylethene-based coordination cages. ChemPhotoChem. 2019;3:378–383. doi: 10.1002/cptc.201900038. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

