Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering
- PMID: 40508766
- PMCID: PMC12157165
- DOI: 10.3390/polym17111520
Antibacterial Crosslinker for Ternary PCL-Reinforced Hydrogels Based on Chitosan, Polyvinyl Alcohol, and Gelatin for Tissue Engineering
Abstract
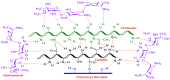
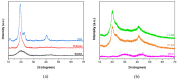
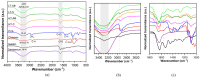
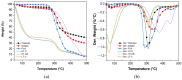
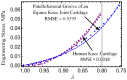
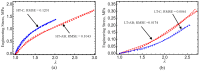
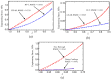
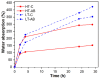
Current hydrogels used for cartilage tissue engineering often lack the mechanical strength and structural integrity required to mimic native human cartilage. This study addresses this limitation by developing reinforced hydrogels based on a ternary polymer blend of poly(vinyl) alcohol (PVA), gelatin (GL), and chitosan (CH), with gentamicin sulfate (GS) as an antimicrobial agent and a crosslinker. The hydrogels were produced using two crosslinking methods, the freeze/thaw and heated cycles, and reinforced with forcespun polycaprolactone (PCL) nanofiber to improve mechanical performance. Chemical characterization revealed that GS forms weak hydrogen bonds with the ternary polymers, leading to esterification with PVA, and covalent bonds are formed as the result of the free amino group (-NH2) of chitosan that reacts with the carboxylic acid group (-COOH) of gelatin. SEM images help us to see how the hydrogels are reinforced with polycaprolactone (PCL) fibers produced via force spinning technology, while mechanical properties were evaluated via uniaxial tensile and compressive tests. Water retention measurements were performed to examine the crosslinking process's influence on the hydrogel's water retention, while the hydrogel surface roughness was obtained via confocal microscopy images. A constitutive model based on non-Gaussian strain energy density was introduced to predict experimental mechanical behavior data of the hydrogel, considering a non-monotonous softening function. Loading and unloading tests demonstrated that GS enhanced crosslinking without compromising water retention or biocompatibility because of the reaction between the free amino group of CH and the carboxylic group of gelatin. The PCL-reinforced PVA/GL/CH hydrogel shows strong potential for cartilage repair and tissue engineering applications.
Keywords: articular cartilage replacement; forcespinning polycaprolactone fibers; gentamicin sulfate; hydrogels; non-Gaussian constitutive material model.
Conflict of interest statement
The authors declare no conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Sodium alginate/chitosan composite scaffold reinforced with biodegradable polyesters/gelatin nanofibers for cartilage tissue engineering.Int J Biol Macromol. 2025 Jan;285:138054. doi: 10.1016/j.ijbiomac.2024.138054. Epub 2024 Nov 27. Int J Biol Macromol. 2025. PMID: 39613057
-
Investigation of Chitosan-Based Hydrogels and Polycaprolactone-Based Electrospun Fibers as Wound Dressing Materials Based on Mechanical, Physical, and Chemical Characterization.Gels. 2025 Jan 4;11(1):39. doi: 10.3390/gels11010039. Gels. 2025. PMID: 39852010 Free PMC article.
-
Hydrogel Based on Chitosan/Gelatin/Poly(Vinyl Alcohol) for In Vitro Human Auricular Chondrocyte Culture.Polymers (Basel). 2024 Feb 8;16(4):479. doi: 10.3390/polym16040479. Polymers (Basel). 2024. PMID: 38399857 Free PMC article.
-
Copper nanoparticles loaded gelatin/ polyvinyl alcohol/ guar gum-based 3D printable multimaterial hydrogel for tissue engineering applications.Int J Biol Macromol. 2024 Sep;276(Pt 1):133866. doi: 10.1016/j.ijbiomac.2024.133866. Epub 2024 Jul 14. Int J Biol Macromol. 2024. PMID: 39009268
-
Magnesium-Doped Nano-Hydroxyapatite/Polyvinyl Alcohol/Chitosan Composite Hydrogel: Preparation and Characterization.Int J Nanomedicine. 2024 Jan 20;19:651-671. doi: 10.2147/IJN.S434060. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38269254 Free PMC article.
Cited by
-
Interpretable Prediction and Analysis of PVA Hydrogel Mechanical Behavior Using Machine Learning.Gels. 2025 Jul 16;11(7):550. doi: 10.3390/gels11070550. Gels. 2025. PMID: 40710711 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

