Genetically Modified Animal-Derived Products: From Regulations to Applications
- PMID: 40509036
- PMCID: PMC12153536
- DOI: 10.3390/ani15111570
Genetically Modified Animal-Derived Products: From Regulations to Applications
Abstract
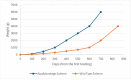
Biotechnological advances applied to the generation of genetically modified (GM) animals have shown the potential to develop innovative solutions for different challenges in key areas such as agriculture and human medicine. Despite its enormous potential, the deployment of genetic modification in animals, and its subsequent commercialization, does not meet the same public acceptance as GM plant-derived products, which are currently widely adopted around the world. In this review, we highlight the main examples of GM and gene-edited animal-derived products already approved by the FDA and discuss the regulatory context inherent to such processes, including the risk-based assessment analysis based on a case-by-case evaluation. Moreover, cases of GM animals already approved by other jurisdictions around the world are also discussed.
Keywords: CRISPR; FDA; GMO; gene-editing; genetic engineering; intentional genomic alterations.
Conflict of interest statement
Tonka Buha was employed by the company SPAROS Lda. The remaining authors declare no conflicts of interest.
Figures

References
-
- Lawrence T.L.J., Fowler V.R., Novakofski J.E. General Aspects of Growth. CABI; Wallingford, UK: 2012. pp. 1–5. - DOI
-
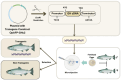
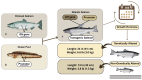
- Fedoroff N., Benfey T., Giddings L.V., Jackson J., Lichatowich J., Lovejoy T., Stanford J., Thurow R.F., Williams R.N. Biotechnology Can Help Us Save the Genetic Heritage of Salmon and Other Aquatic Species. Proc. Natl. Acad. Sci. USA. 2022;119:2–5. doi: 10.1073/pnas.2202184119. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

