Two Species of Long-Day Breeding Hamsters Exhibit Distinct Gut Microbial Responses to Photoperiodic Variations
- PMID: 40509114
- PMCID: PMC12153784
- DOI: 10.3390/ani15111648
Two Species of Long-Day Breeding Hamsters Exhibit Distinct Gut Microbial Responses to Photoperiodic Variations
Abstract
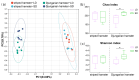
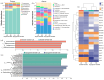
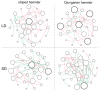
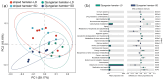
The relationship between the gut microbiota and photoperiod has received widespread attention, and it is necessary to explore the probable common mechanisms involved. We tested whether the gut microbiota of animals with similar light-regulated life history traits would also exhibit consistent responses to the photoperiod. Here, two species of long-day breeders, striped hamsters (Cricetulus barabensis) and Djungarian hamsters (Phodopus sungorus), were raised under different photoperiods (long daylight, LD; short daylight, SD), and their cecal contents were collected to assess the gut microbiota. There was no difference in the gut microbial diversity between the groups of striped hamsters; however, in the Djungarian hamsters, lower Chao and Shannon indices were observed in the LD group than in the SD group. The bacterial community variation in the striped hamsters was reflected mainly in the enrichment of the genera Enterorhabdus and Jeotgalicoccus in the LD group; meanwhile, more taxa with significant changes in relative abundance under different photoperiods were found in the Djungarian hamsters, such as the enrichment of the genera Lactobacillus and Faecalibaculum in the LD group and the enrichment of the genera Ruminococcus and Colidextribacter in the SD group. The LD conditions substantially reduced the complexity of the gut microbial network in the Djungarian hamsters and increased the R2 value of the striped hamster gut microbiota under fitting with a neutral community model. Moreover, the potential gut microbial functions in the striped hamsters were relatively stable, but variations were observed in multiple pathways between the groups of Djungarian hamsters. These results contribute to the understanding of host species specificity in the response of the gut microbiota to external changes.
Keywords: 16S rRNA gene; gut microbiota; photoperiods; rodents; species specific.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Host-Specific Differences in Gut Microbiota Between Cricetulus barabensis and Phodopus sungorus.Curr Microbiol. 2023 Mar 27;80(5):149. doi: 10.1007/s00284-023-03274-4. Curr Microbiol. 2023. PMID: 36971869
-
Enhanced serum oestrogen levels and highly steroidogenic, luteinized atretic follicles in the ovaries of the Djungarian hamster (Phodopus sungorus) kept under a short photoperiod from birth.Eur J Endocrinol. 2002 Nov;147(5):701-10. doi: 10.1530/eje.0.1470701. Eur J Endocrinol. 2002. PMID: 12444903
-
Different gut microbial types were found in captive striped hamsters.PeerJ. 2023 Nov 6;11:e16365. doi: 10.7717/peerj.16365. eCollection 2023. PeerJ. 2023. PMID: 37953783 Free PMC article.
-
Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus.J Comp Physiol B. 2000 Feb;170(1):37-43. doi: 10.1007/s003600050005. J Comp Physiol B. 2000. PMID: 10707323
-
Short-day response in Djungarian hamsters of different circadian phenotypes.Chronobiol Int. 2012 May;29(4):430-42. doi: 10.3109/07420528.2012.668506. Chronobiol Int. 2012. PMID: 22515562
References
-
- Fragiadakis G.K., Smits S.A., Sonnenburg E.D., Van Treuren W., Reid G., Knight R., Manjurano A., Changalucha J., Dominguez-Bello M.G., Leach J., et al. Links between environment, diet, and the hunter-gatherer microbiome. Gut Microbes. 2019;10:216–227. doi: 10.1080/19490976.2018.1494103. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

