Influence of Hydrolysis on Non-Isothermal Crystallization of Poly(Butylene Succinate-Co-Adipate) (PBSA)
- PMID: 40509142
- PMCID: PMC12155848
- DOI: 10.3390/molecules30112252
Influence of Hydrolysis on Non-Isothermal Crystallization of Poly(Butylene Succinate-Co-Adipate) (PBSA)
Abstract
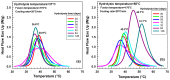
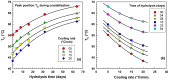
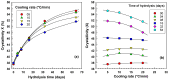
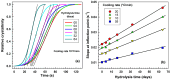
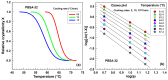
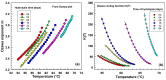
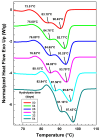
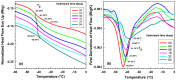
This study investigates the impact of hydrolysis on the crystallization behavior of poly(butylene succinate-co-adipate) (PBSA), a biodegradable polyester. Hydrolysis was conducted in a controlled environment using phosphate-buffered saline at 70 °C to isolate the impact of hydrolytic degradation on the polymer's properties. The consequent changes in molecular weight characteristics were tracked using gel permeation chromatography (GPC), revealing a decrease in both weight average molecular weight (Mw) and an increase in polydispersity index (PDI) as hydrolysis progressed. The thermal behavior of PBSA during hydrolysis was thoroughly investigated using differential scanning calorimetry (DSC), which demonstrated significant changes in melting temperature (Tm), glass transition temperature (Tg), and crystallinity (X). These changes in Tm and Tg suggest a change in copolymer composition, likely due to the greater susceptibility of the adipic acid unit to hydrolysis compared to the succinic acid unit. Furthermore, polarized optical microscopy (POM) was employed to observe the morphological evolution of PBSA, showing a transition from spherulitic structures in the early stages of hydrolysis to dendritic structures with prolonged hydrolysis time. The decrease in nucleation activity led to a reduction in the number of spherulites, which in turn allowed the remaining spherulites to grow larger.
Keywords: biodegradable polyesters; crystallization kinetics; crystallization morphology; degradation; hydrolysis; molecular weight distribution; non-isothermal crystallization; poly(butylene succinate-co-adipate) (PBSA); spherulites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Padermshoke A., An Y.J., Kajiwara T., Masunaga H., Kobayashi Y., Ito H., Sasaki S., Noguchi H., Kusuno A., Takahara A. Photooxidation-induced conformational changes and degradation behaviors of poly(butylene succinate) and poly(butylene succinate-adipate) J. Polym. Sci. 2024;62:3794–3807. doi: 10.1002/pol.20230911. - DOI
-
- Verma S.K., Prasad A., Sonika, Katiyar V. State of art review on sustainable biodegradable polymers with a market overview for sustainability packaging. Mater. Today Sustain. 2024;26:100776. doi: 10.1016/j.mtsust.2024.100776. - DOI
-
- Prieur M., Sudre G., Gouanvé F., Fulchiron R., Espuche E. Effect of mechanical stretching and low amount of silica adding on gas transport properties of poly(butylene succinate-co-adipate) films. Polymer. 2024;304:127135. doi: 10.1016/j.polymer.2024.127135. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

