Antibody Aggregate Removal by Multimodal Chromatography
- PMID: 40509250
- PMCID: PMC12156806
- DOI: 10.3390/molecules30112363
Antibody Aggregate Removal by Multimodal Chromatography
Abstract
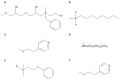
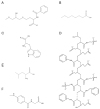
The growing demand for therapeutic monoclonal antibodies (mAbs) has heightened the need for efficient and scalable purification strategies. A major challenge in downstream processing is the removal of antibody aggregates, which can compromise drug safety, efficacy, and regulatory compliance. This review explores the use of multimodal chromatography for aggregate separation, providing an in-depth analysis of commercially available resins and emerging adsorbent prototypes. It also examines the mechanisms of aggregate formation during bioprocessing. A comparative evaluation of conventional single-mode chromatography techniques-affinity, ion exchange, and hydrophobic interaction-is presented alongside multimodal chromatography, which integrates ion-exchange, hydrophobic, and other non-covalent interactions for enhanced aggregate clearance and process flexibility. The review primarily assesses commercial multimodal resins in terms of aggregate removal efficiency, binding capacity, and scalability. Additionally, advancements in prototype resins and multimodal membranes are discussed. Finally, the advantages, limitations, and future directions of multimodal chromatography in mAb aggregate removal are outlined. As purification demands continue to evolve, multimodal chromatography is poised to play an increasingly critical role in achieving the high purity standards required for therapeutic antibodies.
Keywords: aggregate formation; antibody aggregates; hydrophobic interaction chromatography; ion-exchange chromatography; mAb purification; multimodal chromatography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- The Antibody Society . Antibody Therapeutics in Late-Stage Clinical Studies. The Antibody Society; Framingham, MA, USA: 2024.
-
- Whitfield K. In Vitro and In Vivo Monoclonal Antibody Production. [(accessed on 28 May 2025)]. Available online: https://pivotalscientific.com/scientific-library/in-vitro-and-in-vivo-mo...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

