Tailored Carbon Nanocomposites for Efficient CO2 Capture
- PMID: 40509295
- PMCID: PMC12156297
- DOI: 10.3390/molecules30112408
Tailored Carbon Nanocomposites for Efficient CO2 Capture
Abstract
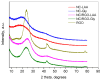
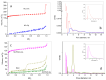
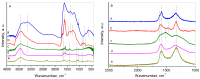
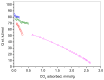
CO2 capture by adsorption on proper solid materials appears to be a promising approach, due to its low energy requirements and ease of implementation. This study aimed to prepare efficient materials for CO2 capture based on composites of nanocarbon and reduced graphene oxide, using graphite, L-ascorbic acid, and glycine as precursors. The materials were characterized by XRD, low-temperature N2 adsorption, FTIR, Raman, and XPS spectroscopies, along with SEM and TEM. The CO2 adsorption capacities, heats of adsorption, and selectivity were determined. A hierarchical porous structure was found for NC-LAA, NC/RGO-LAA, and NC/RGO-Gly. At 273 K and 100 kPa, the adsorption capacities for NC-LAA and NC-Gly reached 2.6 mmol/g and 2.5 mmol/g, respectively, while for the composites, the capacities were 1.7 mmol/g for NC/RGO-Gly and 3.5 mmol/g for NC/RGO-LAA. The adsorption ability of the glycine-derived materials is related to the presence of nitrogen-containing functional groups. The heats of adsorption for NC-LAA, NC-Gly, and NC/RGO-Gly reveal chemisorption with CO2. Except for chemisorption, the NC/RGO-LAA material shows a sustained physical adsorption up to higher CO2 coverage. The best adsorption of CO2, observed for NC/RGO-LAA, is connected with the synergy between carbon dots and RGO. This composition ensures both sufficient oxygen surface functionalization and a proper hierarchical porous structure.
Keywords: CO2 adsorption; carbon dots; nanocarbon; nanocomposite; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption.Polymers (Basel). 2020 Sep 17;12(9):2117. doi: 10.3390/polym12092117. Polymers (Basel). 2020. PMID: 32957497 Free PMC article.
-
Reduced graphene oxide -MnO2 nanocomposite for CO2 capture from flue gases at elevated temperatures.Sci Total Environ. 2022 Apr 10;816:151522. doi: 10.1016/j.scitotenv.2021.151522. Epub 2021 Nov 6. Sci Total Environ. 2022. PMID: 34752862
-
Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption.Molecules. 2021 Aug 31;26(17):5293. doi: 10.3390/molecules26175293. Molecules. 2021. PMID: 34500736 Free PMC article.
-
Three different methods for ZnO-RGO nanocomposite synthesis and its adsorption capacity for methylene blue dye removal in a comparative study.BMC Chem. 2025 Jan 18;19(1):18. doi: 10.1186/s13065-025-01381-w. BMC Chem. 2025. PMID: 39827167 Free PMC article.
-
Critical assessment of the performance of next-generation carbon-based adsorbents for CO2 capture focused on their structural properties.Sci Total Environ. 2022 Mar 1;810:151720. doi: 10.1016/j.scitotenv.2021.151720. Epub 2021 Nov 30. Sci Total Environ. 2022. PMID: 34861307 Review.
References
-
- Sayari A., Belmabkhout Y., Serna-Guerero R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011;171:760–774. doi: 10.1016/j.cej.2011.02.007. - DOI
-
- Maina J.W., Pozo-Gonzalo C., Kong L., Schütz J., Hill M., Dumée L.F. Metal organic framework based catalysts for CO2 conversion. Mater. Horiz. 2017;4:345–361. doi: 10.1039/C6MH00484A. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

