Exogenous L-Cysteine and Its Transport Through CtaP Play a Role in Biofilm Formation, Swimming Motility, and Swarming Motility of Listeria monocytogenes
- PMID: 40509373
- PMCID: PMC12155340
- DOI: 10.3390/foods14111845
Exogenous L-Cysteine and Its Transport Through CtaP Play a Role in Biofilm Formation, Swimming Motility, and Swarming Motility of Listeria monocytogenes
Abstract
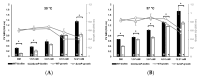
Listeria monocytogenes is of a significant concern for the food industry, largely due to its ability to form biofilms. Flagellar motility and environmental factors are crucial for biofilm formation. Cysteine is an important compound affecting the behavior of this bacterium; therefore, we investigated its role in growth, biofilm formation and motility of L. monocytogenes 10403S through a mutant in cysteine uptake (ΔctaP). Basal defined media (DM) and L-cysteine-supplemented DM were used. Biofilm formation was promoted by L-cysteine supplementation in both wild type (WT) and ΔctaP. Lower biofilm formation of ΔctaP compared to WT indicates the significance of the cysteine transporter and cysteine uptake. A negative correlation was found between growth and biofilm formation, especially in the presence of high L-cysteine concentrations. Motility experiments showed that as the L-cysteine concentration increased, the swarming motility of WT decreased. Furthermore, swimming motility of WT was enhanced with L-cysteine supplementation, while the swimming motility of ΔctaP remained unaffected. To evaluate the role of cysteine and CtaP in biofilm formation and motility, transcriptome analysis, comparing WT and ΔctaP in basal and L-cysteine-supplemented (1.57 and 3.67 mM) DM, was conducted at 37 °C. The investigation of biofilm-related genes explained the role of ctaP and revealed induced expression of flagella and chemotaxis genes by L-cysteine.
Keywords: Listeria monocytogenes; bacterial adhesion; ctaP; cysteine transporter; environmental sensing; flagellar motility; quorum sensing.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Quereda J.J., Morón-García A., Palacios-Gorba C., Dessaux C., García-del Portillo F., Pucciarelli M.G., Ortega A.D. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence. 2021;12:2509–2545. doi: 10.1080/21505594.2021.1975526. - DOI - PMC - PubMed
-
- Ravindhiran R., Sivarajan K., Sekar J.N., Murugesan R., Dhandapani K. Listeria monocytogenes an emerging pathogen: A comprehensive overview on listeriosis, virulence determinants, detection, and anti-listerial interventions. Microb. Ecol. 2023;86:2231–2251. doi: 10.1007/s00248-023-02269-9. - DOI - PubMed
-
- Su Y., Liu A., Zhu M.J. Mapping the landscape of listeriosis outbreaks (1998–2023): Trends, challenges, and regulatory responses in the United States. Trends Food Sci. Technol. 2024;154:104750. doi: 10.1016/j.tifs.2024.104750. - DOI
LinkOut - more resources
Full Text Sources

