Integrin Alpha8 Beta1 (81): An In-Depth Review of an Overlooked RGD-Binding Receptor
- PMID: 40510035
- PMCID: PMC12162094
- DOI: 10.32604/biocell.2025.062325
Integrin Alpha8 Beta1 (81): An In-Depth Review of an Overlooked RGD-Binding Receptor
Abstract
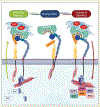
Integrins are heterodimeric transmembrane receptors that mediate bidirectional interactions between the intracellular cytoskeletal array and the extracellular matrix. These interactions are critical in tissue development and function by regulating gene expression and sustaining tissue architecture. In humans, the integrin family is composed of 18 alpha (α) and 8 beta (β) subunits, constituting 24 distinct αβ combinations. Based on their structure and ligand-binding properties, only a subset of integrins, 8 out of 24, recognizes the arginine-glycine-aspartate (RGD) tripeptide motif in the native ligand. One of the major RGD binding integrins is integrin alpha 8 beta 1 (α8β1), a central Ras homolog gene family member A (RHOA)-dependent modulator highly expressed in cells with contractile function. This review focuses on the recent advances regarding α8β1 function during organ development, with a particular interest in kidney and inner ear development. We also discuss α8β1's role in injury and disease and its importance for mesenchymal to epithelial transition during cancer development. Finally, we highlight α8β1's importance for hearing function and its future use as a potential diagnostic and therapeutic tool for disease elimination.
Keywords: RGD binding integrin; Transmembrane receptor; cytoskeleton.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Figures



References
-
- Gravina AN, D’Elia ND, Benedini LA, Messina P. A commentary on the interplay of biomaterials and cell adhesion: new insights in bone tissue regeneration. BIOCELL. 2024;48(11):1517–20. doi: 10.32604/biocell.2024.055513. - DOI
-
- Wen L, Lee S, Ley K. A splice variant of kindlin-3 is functional in β2 integrin activation and neutrophil adhesion. J Immunol. 2024;212(1_Supplement):1156–5049. doi: 10.4049/jimmunol.212.supp.1156.5049. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
