The landscape and clinical impact of tumor-associated macrophages and PD-L1 in primary breast cancers and their brain metastases
- PMID: 40510346
- PMCID: PMC12159042
- DOI: 10.3389/fimmu.2025.1598293
The landscape and clinical impact of tumor-associated macrophages and PD-L1 in primary breast cancers and their brain metastases
Abstract
Background: Tumor-associated macrophages (TAMs) influence the tumor microenvironment and can contribute to tumor progression. They can polarize into M1 (classically activated) or M2 (alternatively activated) phenotype, which exhibit divergent functional characteristics. The comparison of TAMs between primary breast cancer (BC) and corresponding brain metastases (BMs) remains insufficiently explored and is the focus of this study.
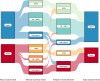
Methods: This study aimed to compare the infiltration of TAMs and PD-L1 expression in primary breast cancer and their brain metastases, by analyzing 27 paired samples and 26 additional brain metastases. Immunohistochemical staining was performed for the following markers: CD68, CD86 (M1), CD163 (M2), and PD-L1.
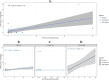
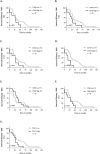
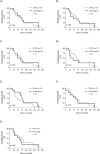
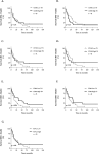
Results: CD68 showed significantly higher expression levels in brain metastases compared to the corresponding primary breast cancers. In contrast, the expression of CD86 and CD163 showed comparable results between the primary tumors and their brain metastatic counterparts. Macrophages were consistently found to be more frequently present in the tumor stroma compared to the tumor nest. Survival analysis of the primary revealed that high expression of CD163 was associated with a recurrence-free survival. (RFS). Conversely, high expression of CD86 in brain metastases was associated with longer overall survival. Low expression of CD68 and CD163 in brain metastases correlated with the presence of meningeal carcinomatosis. The expression of PD-L1 in the primary tumor did not necessarily reflect the status of PD-L1 in the corresponding brain metastases.
Conclusions: Overall, this study highlights the complex influence of TAMs on the course of primary breast cancers and their brain metastases. The discordant expression of the immune checkpoint molecule PD-L1 underscores the importance of evaluating the PD-L1 status in cerebral metastases to guide potential immunotherapeutic approaches.
Keywords: CD163; CD68; CD86; PD-L1; TAMs; brain metastases; breast cancer; tumor-associated macrophages.
Copyright © 2025 Zimmer, Hanke, Neyazi, Rashidi, Schaufler, Dumitru, Ignatov, Mawrin, Sandalcioglu and Stein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CD68 positive and/or CD163 positive tumor-associated macrophages and PD-L1 expression in breast phyllodes tumor.Breast Cancer Res Treat. 2025 Jan;209(2):261-273. doi: 10.1007/s10549-024-07487-4. Epub 2024 Sep 6. Breast Cancer Res Treat. 2025. PMID: 39242456
-
M2-type tumor-associated macrophages upregulated PD-L1 expression in cervical cancer via the PI3K/AKT pathway.Eur J Med Res. 2024 Jul 5;29(1):357. doi: 10.1186/s40001-024-01897-2. Eur J Med Res. 2024. PMID: 38970071 Free PMC article.
-
The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients.BMC Cancer. 2012 Jul 23;12:306. doi: 10.1186/1471-2407-12-306. BMC Cancer. 2012. PMID: 22824040 Free PMC article.
-
Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis.Oral Oncol. 2019 Jun;93:66-75. doi: 10.1016/j.oraloncology.2019.04.019. Epub 2019 Apr 28. Oral Oncol. 2019. PMID: 31109698
-
Prognostic value of CD163+ macrophages in solid tumor malignancies: A scoping review.Immunol Lett. 2025 Apr;272:106970. doi: 10.1016/j.imlet.2025.106970. Epub 2025 Jan 6. Immunol Lett. 2025. PMID: 39778658
References
-
- Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. (2019) 121:991–1000. doi: 10.1038/s41416-019-0619-y - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

