The efficacy and safety of regorafenib/fruquintinib combined with PD-1/PD-L1 for metastatic colorectal cancer: a meta-analysis based on single-arm studies
- PMID: 40510355
- PMCID: PMC12159013
- DOI: 10.3389/fimmu.2025.1579293
The efficacy and safety of regorafenib/fruquintinib combined with PD-1/PD-L1 for metastatic colorectal cancer: a meta-analysis based on single-arm studies
Abstract
Objective: The efficacy of regorafenib or fruquintinib in combination with PD-1/PD-L1 inhibitors for metastatic colorectal cancer (mCRC) treatment has not been elucidated. This study aims to systematically evaluate the efficacy and safety of this combination therapy.
Methods: PubMed, Embase, Cochrane Library, and Web of Science were systematically retrieved until July 24, 2024. A meta-analysis was carried out for the overall objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and the incidence of grade 3 or higher treatment-related adverse events (AEs). Non-overlapping 95% confidence intervals (CIs) were considered statistically significant.
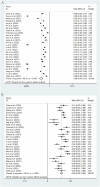
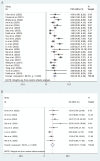
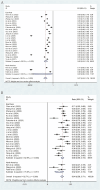
Results: 26 studies encompassing 1,409 patients were analyzed. Pooled analysis revealed an ORR of 6% (95% CI: 3%-12%), a DCR of 62% (95% CI: 55%-68%), a median PFS of 3.84 months (95% CI: 3.19-4.49 months), a median OS of 13.08 months (95% CI: 10.17-16.00 months), and an incidence rate of grade 3-4 AEs of 21% (95% CI: 15%-28%). In subgroup analyses, the fruquintinib-based regimen demonstrated significantly superior efficacy compared to regorafenib-based therapy, with higher ORR (16% [95% CI: 13%-21%] vs 3% [95% CI: 1%-9%]), DCR (79% [95% CI: 72%-85%] vs 54% [95% CI: 47%-61%]), and median PFS (5.40 months [95% CI: 4.60-6.19] vs 3.00 months [95% CI: 2.47-3.52]). Median OS was numerically but not significantly longer with fruquintinib (14.35 months [95% CI: 10.68-18.02] vs 12.70 months [95% CI: 8.79-16.61]). Liver metastasis status strongly influenced outcomes, with significantly lower ORR (3% [95% CI: 1%-13%] vs 49% [95% CI: 32%-76%]) and shorter median PFS (2.37 months [95% CI: 1.77-2.96] vs 3.50 months [95% CI: 3.09-3.91]) in patients with liver involvement.
Conclusion: The combination of regorafenib or fruquintinib with PD-1/PD-L1 shows moderate efficacy and acceptable safety in the treatment of mCRC. The fruquintinib-based regimen may be superior to the regorafenib-based regimen, and patients without liver metastasis may derive greater benefits. These findings offer new insights for treating mCRC, although they should be validated through large randomized controlled trials.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42024582268.
Keywords: PD-1/PD-L1 inhibitors; fruquintinib; meta-analysis; metastatic colorectal cancer; regorafenib.
Copyright © 2025 Yang, Mao, Huang, Luo, Liu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
A comparison of regorafenib and fruquintinib for metastatic colorectal cancer: a systematic review and network meta-analysis.J Cancer Res Clin Oncol. 2019 Sep;145(9):2313-2323. doi: 10.1007/s00432-019-02964-6. Epub 2019 Jul 5. J Cancer Res Clin Oncol. 2019. PMID: 31278474 Free PMC article.
-
Comparison of Efficacy and Safety of Single and Double Immune Checkpoint Inhibitor-Based First-Line Treatments for Advanced Driver-Gene Wild-Type Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis.Front Immunol. 2021 Aug 16;12:731546. doi: 10.3389/fimmu.2021.731546. eCollection 2021. Front Immunol. 2021. PMID: 34484242 Free PMC article.
-
Real-world comparison of chemotherapy plus bevacizumab with or without immunotherapy as first-line therapy in colorectal cancer.World J Gastroenterol. 2025 Jun 28;31(24):108298. doi: 10.3748/wjg.v31.i24.108298. World J Gastroenterol. 2025. PMID: 40599189 Free PMC article.
-
Efficacy of PD-1/PDL-1 inhibitors for ovarian cancer: a systematic review and network meta-analysis.Future Oncol. 2025 Aug;21(20):2637-2647. doi: 10.1080/14796694.2025.2530378. Epub 2025 Jul 12. Future Oncol. 2025. PMID: 40650991
References
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. (2013) 381:303–12. doi: 10.1016/s0140-6736(12)61900-x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

