Targeting programmed cell death with natural products: a potential therapeutic strategy for diminished ovarian reserve and fertility preservation
- PMID: 40510420
- PMCID: PMC12158948
- DOI: 10.3389/fphar.2025.1546041
Targeting programmed cell death with natural products: a potential therapeutic strategy for diminished ovarian reserve and fertility preservation
Abstract
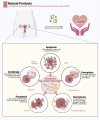
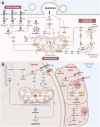
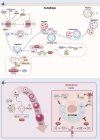
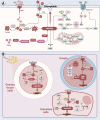
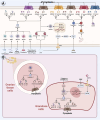
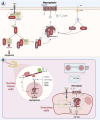
The depletion of ovarian reserve is a major factor contributing to the decline in female fertility. It is characterized by a simultaneous reduction in the quantity and quality of oocytes and the follicular pools. The cyclic recruitment of primordial follicles and the preservation of oocyte quality involve complex and tightly regulated biological processes. Granulosa cells, which surround the oocytes, play a pivotal role in follicular development and the determination of follicular fate. Programmed cell death (PCD), a genetically regulated process of cell elimination, is a key factor in the regulation of ovarian reserve dynamics. Emerging evidence suggests that natural products derived from medicinal plants, dietary components, animals, and microorganisms may modulate PCD in granulosa cells through various molecular mechanisms and signaling pathways. These natural products have demonstrated preliminary effects in delaying ovarian aging and preserving ovarian reserve in preclinical models. This review discusses the roles and underlying mechanisms of various forms of PCD in diminished ovarian reserve, while summarizing the current findings on natural products that influence granulosa cells PCD to protect ovarian function. These insights may contribute to the future development of novel, targeted strategies aimed at preserving female reproductive potential.
Keywords: IVF; diminished ovarian reserve; female fertility; natural products; programmed cell death.
Copyright © 2025 Ju, Zhao, Li, Zhang, Xiang and Lian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model.Hum Reprod. 2021 Aug 18;36(9):2514-2528. doi: 10.1093/humrep/deab165. Hum Reprod. 2021. PMID: 34333622 Free PMC article.
-
Upregulated let-7 expression in the follicular fluid of patients with endometriomas leads to dysfunction of granulosa cells through targeting of IGF1R.Hum Reprod. 2025 Jan 1;40(1):119-137. doi: 10.1093/humrep/deae247. Hum Reprod. 2025. PMID: 39521729 Free PMC article.
-
Ovarian aging-associated downregulation of GPX4 expression regulates ovarian follicular development by affecting granulosa cell functions and oocyte quality.FASEB J. 2025 Mar 31;39(6):e70469. doi: 10.1096/fj.202401580RR. FASEB J. 2025. PMID: 40100097
-
A matter of new life and cell death: programmed cell death in the mammalian ovary.J Biomed Sci. 2024 Mar 20;31(1):31. doi: 10.1186/s12929-024-01017-6. J Biomed Sci. 2024. PMID: 38509545 Free PMC article. Review.
-
The impact of mitochondrial dysfunction on ovarian aging.J Transl Med. 2025 Feb 20;23(1):211. doi: 10.1186/s12967-025-06223-w. J Transl Med. 2025. PMID: 39980008 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

